Label: NORPACE- disopyramide phosphate capsule, gelatin coated
NORPACE CR- disopyramide phosphate capsule, extended release
- NDC Code(s): 0025-2732-31, 0025-2742-31, 0025-2752-31, 0025-2762-31
- Packager: Pfizer Laboratories Div Pfizer Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
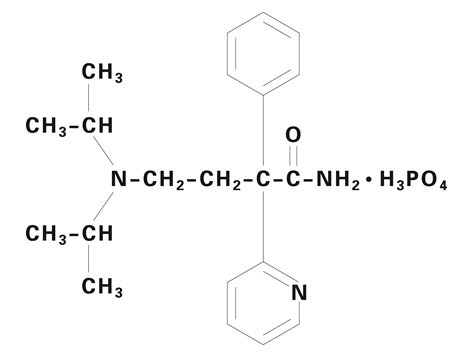
Norpace (disopyramide phosphate) is an antiarrhythmic drug available for oral administration in immediate-release and controlled-release capsules containing 100 mg or 150 mg of disopyramide base, present as the phosphate. The base content of the phosphate salt is 77.6%. The structural formula of Norpace is:
Norpace is freely soluble in water, and the free base (pKa 10.4) has an aqueous solubility of 1 mg/ml. The chloroform:water partition coefficient of the base is 3.1 at pH 7.2.
Norpace is a racemic mixture of d- and l- isomers. This drug is not chemically related to other antiarrhythmic drugs.
Norpace CR (controlled-release) capsules are designed to afford a gradual and consistent release of disopyramide. Thus, for maintenance therapy, Norpace CR provides the benefit of less-frequent dosing (every 12 hours) as compared with the every-6-hour dosage schedule of immediate-release Norpace capsules.
Inactive ingredients of Norpace include corn starch, edible ink, FD&C Red No. 3, FD&C Yellow No. 6, gelatin, lactose, talc, and titanium dioxide; the 150-mg capsule also contains FD&C Blue No. 1.
Inactive ingredients of Norpace CR include corn starch, D&C Yellow No. 10, edible ink, ethylcellulose, FD&C Blue No. 1, gelatin, shellac, sucrose, talc, and titanium dioxide; the 150-mg capsule also contains FD&C Red No. 3 and FD&C Yellow No. 6.
-
CLINICAL PHARMACOLOGY
Mechanisms of Action
Norpace (disopyramide phosphate) is a Type 1 antiarrhythmic drug (i.e., similar to procainamide and quinidine). In animal studies Norpace decreases the rate of diastolic depolarization (phase 4) in cells with augmented automaticity, decreases the upstroke velocity (phase 0) and increases the action potential duration of normal cardiac cells, decreases the disparity in refractoriness between infarcted and adjacent normally perfused myocardium, and has no effect on alpha- or beta-adrenergic receptors.
Electrophysiology
In man, Norpace at therapeutic plasma levels shortens the sinus node recovery time, lengthens the effective refractory period of the atrium, and has a minimal effect on the effective refractory period of the AV node. Little effect has been shown on AV-nodal and His-Purkinje conduction times or QRS duration. However, prolongation of conduction in accessory pathways occurs.
Hemodynamics
At recommended oral doses, Norpace rarely produces significant alterations of blood pressure in patients without congestive heart failure (see Warnings). With intravenous Norpace, either increases in systolic/diastolic or decreases in systolic blood pressure have been reported, depending on the infusion rate and the patient population. Intravenous Norpace may cause cardiac depression with an approximate mean 10% reduction of cardiac output, which is more pronounced in patients with cardiac dysfunction.
Anticholinergic Activity
The in vitro anticholinergic activity of Norpace is approximately 0.06% that of atropine; however, the usual dose for Norpace is 150 mg every 6 hours and for Norpace CR 300 mg every 12 hours, compared to 0.4 to 0.6 mg for atropine (see Warnings and Adverse Reactions for anticholinergic side effects).
Pharmacokinetics
Following oral administration of immediate-release Norpace, disopyramide phosphate is rapidly and almost completely absorbed, and peak plasma levels are usually attained within 2 hours. The usual therapeutic plasma levels of disopyramide base are 2 to 4 mcg/ml, and at these concentrations protein binding varies from 50% to 65%. Because of concentration-dependent protein binding, it is difficult to predict the concentration of the free drug when total drug is measured.
The mean plasma half-life of disopyramide in healthy humans is 6.7 hours (range of 4 to 10 hours). In six patients with impaired renal function (creatinine clearance less than 40 ml/min), disopyramide half-life values were 8 to 18 hours.
After the oral administration of 200 mg of disopyramide to 10 cardiac patients with borderline to moderate heart failure, the time to peak serum concentration of 2.3 ± 1.5 hours (mean ± SD) was increased, and the mean peak serum concentration of 4.8 ± 1.6 mcg/ml was higher than in healthy volunteers. After intravenous administration in these same patients, the mean elimination half-life was 9.7 ± 4.2 hours (range in healthy volunteers of 4.4 to 7.8 hours). In a second study of the oral administration of disopyramide to 7 patients with heart disease, including left ventricular dysfunction, the mean plasma half-life was slightly prolonged to 7.8 ± 1.9 hours (range of 5 to 9.5 hours).
In healthy men, about 50% of a given dose of disopyramide is excreted in the urine as the unchanged drug, about 20% as the mono-N-dealkylated metabolite, and 10% as the other metabolites. The plasma concentration of the major metabolite is approximately one tenth that of disopyramide. Altering the urinary pH in man does not affect the plasma half-life of disopyramide.
In a crossover study in healthy subjects, the bio-availability of disopyramide from Norpace CR capsules was similar to that from the immediate-release capsules. With a single 300-mg oral dose, peak disopyramide plasma concentrations of 3.23 ± 0.75 mcg/ml (mean ± SD) at 2.5 ± 2.3 hours were obtained with two 150-mg immediate-release capsules and 2.22 ± 0.47 mcg/ml at 4.9 ± 1.4 hours with two 150-mg Norpace CR capsules. The elimination half-life of disopyramide was 8.31 ± 1.83 hours with the immediate-release capsules and 11.65 ± 4.72 hours with Norpace CR capsules. The amount of disopyramide and mono-N-dealkylated metabolite excreted in the urine in 48 hours was 128 and 48 mg, respectively, with the immediate-release capsules, and 112 and 33 mg, respectively, with Norpace CR capsules. The differences in the urinary excretion of either constituent were not statistically significant.
Following multiple doses, steady-state plasma levels of between 2 and 4 mcg/ml were attained following either 150 mg every-6-hour dosing with immediate-release capsules or 300 mg every-12-hour dosing with Norpace CR capsules.
Drug Interactions
Effects of other drugs on disopyramide pharmacokinetics: In vitro metabolic studies indicated that disopyramide is metabolized by cytochrome P450 3A4 and that inhibitors of this enzyme may result in elevation of plasma levels of disopyramide. Although specific drug interaction studies have not been done, cases of life-threatening interactions have been reported for disopyramide when given with clarithromycin and erythromycin.
-
INDICATIONS AND USAGE
Norpace and Norpace CR are indicated for the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgment of the physician, are life-threatening. Because of the proarrhythmic effects of Norpace and Norpace CR, their use with lesser arrhythmias is generally not recommended. Treatment of patients with asymptomatic ventricular premature contractions should be avoided.
Initiation of Norpace or Norpace CR treatment, as with other antiarrhythmic agents used to treat life-threatening arrhythmias, should be carried out in the hospital. Norpace CR should not be used initially if rapid establishment of disopyramide plasma levels is desired.
Antiarrhythmic drugs have not been shown to enhance survival in patients with ventricular arrhythmias.
- CONTRAINDICATIONS
-
Warnings
Mortality
In the National Heart, Lung and Blood Institute's Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had had a myocardial infarction more than 6 days but less than 2 years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to carefully matched placebo-treated groups (3.0%). The average duration of treatment with encainide or flecainide in this study was 10 months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain. Considering the known proarrhythmic properties of Norpace or Norpace CR and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Norpace or Norpace CR as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias.
Negative Inotropic Properties:
Heart Failure/Hypotension
Norpace or Norpace CR may cause or worsen congestive heart failure or produce severe hypotension as a consequence of its negative inotropic properties. Hypotension has been observed primarily in patients with primary cardiomyopathy or inadequately compensated congestive heart failure. Norpace or Norpace CR should not be used in patients with uncompensated or marginally compensated congestive heart failure or hypotension unless the congestive heart failure or hypotension is secondary to cardiac arrhythmia. Patients with a history of heart failure may be treated with Norpace or Norpace CR, but careful attention must be given to the maintenance of cardiac function, including optimal digitalization. If hypotension occurs or congestive heart failure worsens, Norpace or Norpace CR should be discontinued and, if necessary, restarted at a lower dosage only after adequate cardiac compensation has been established.
QRS Widening
Although it is unusual, significant widening (greater than 25%) of the QRS complex may occur during Norpace or Norpace CR administration; in such cases Norpace or Norpace CR should be discontinued.
Q-T Prolongation
As with other Type 1 antiarrhythmic drugs, prolongation of the Q-T interval (corrected) and worsening of the arrhythmia, including ventricular tachycardia and ventricular fibrillation, may occur. Patients who have evidenced prolongation of the Q-T interval in response to quinidine may be at particular risk. As with other Type 1A antiarrhythmics, disopyramide phosphate has been associated with torsade de pointes.
If a Q-T prolongation of greater than 25% is observed and if ectopy continues, the patient should be monitored closely, and consideration given to discontinuing Norpace or Norpace CR.
Hypoglycemia
In rare instances significant lowering of blood-glucose values has been reported during Norpace administration. The physician should be alert to this possibility, especially in patients with congestive heart failure, chronic malnutrition, hepatic, renal or other diseases, or drugs (e.g., beta-adrenoceptor blockers, alcohol) which could compromise preservation of the normal glucoregulatory mechanisms in the absence of food. In these patients the blood-glucose levels should be carefully followed.
Concomitant Antiarrhythmic Therapy
The concomitant use of Norpace or Norpace CR with other Type 1A antiarrhythmic agents (such as quinidine or procainamide), Type 1C antiarrhythmics (such as encainide, flecainide or propafenone), and/or propranolol should be reserved for patients with life-threatening arrhythmias who are demonstrably unresponsive to single-agent antiarrhythmic therapy. Such use may produce serious negative inotropic effects, or may excessively prolong conduction. This should be considered particularly in patients with any degree of cardiac decompensation or those with a prior history thereof. Patients receiving more than one antiarrhythmic drug must be carefully monitored.
Heart Block
If first-degree heart block develops in a patient receiving Norpace or Norpace CR, the dosage should be reduced. If the block persists despite reduction of dosage, continuation of the drug must depend upon weighing the benefit being obtained against the risk of higher degrees of heart block. Development of second- or third-degree AV block or unifascicular, bifascicular, or trifascicular block requires discontinuation of Norpace or Norpace CR therapy, unless the ventricular rate is adequately controlled by a temporary or implanted ventricular pacemaker.
Anticholinergic Activity
Because of its anticholinergic activity, disopyramide phosphate should not be used in patients with glaucoma, myasthenia gravis, or urinary retention unless adequate overriding measures are taken; these consist of the topical application of potent miotics (e.g., pilocarpine) for patients with glaucoma, and catheter drainage or operative relief for patients with urinary retention. Urinary retention may occur in patients of either sex as a consequence of Norpace or Norpace CR administration, but males with benign prostatic hypertrophy are at particular risk. In patients with a family history of glaucoma, intraocular pressure should be measured before initiating Norpace or Norpace CR therapy. Disopyramide phosphate should be used with special care in patients with myasthenia gravis since its anticholinergic properties could precipitate a myasthenic crisis in such patients.
-
PRECAUTIONS
General
Atrial Tachyarrhythmias
Patients with atrial flutter or fibrillation should be digitalized prior to Norpace or Norpace CR administration to ensure that drug-induced enhancement of AV conduction does not result in an increase of ventricular rate beyond physiologically acceptable limits.
Conduction Abnormalities
Care should be taken when prescribing Norpace or Norpace CR for patients with sick sinus syndrome (bradycardia-tachycardia syndrome), Wolff-Parkinson-White syndrome (WPW), or bundle branch block. The effect of disopyramide phosphate in these conditions is uncertain at present.
Cardiomyopathy
Patients with myocarditis or other cardiomyopathy may develop significant hypotension in response to the usual dosage of disopyramide phosphate, probably due to cardiodepressant mechanisms. Therefore, a loading dose of Norpace should not be given to such patients, and initial dosage and subsequent dosage adjustments should be made under close supervision (see Dosage and Administration).
Renal Impairment
More than 50% of disopyramide is excreted in the urine unchanged. Therefore Norpace dosage should be reduced in patients with impaired renal function (see Dosage and Administration). The electrocardiogram should be carefully monitored for prolongation of PR interval, evidence of QRS widening, or other signs of overdosage (see Overdosage).
Norpace CR is not recommended for patients with severe renal insufficiency (creatinine clearance 40 ml/min or less).
Hepatic Impairment
Hepatic impairment also causes an increase in the plasma half-life of disopyramide. Dosage should be reduced for patients with such impairment. The electrocardiogram should be carefully monitored for signs of overdosage (see Overdosage).
Patients with cardiac dysfunction have a higher potential for hepatic impairment; this should be considered when administering Norpace or Norpace CR.
Drug Interactions
If phenytoin or other hepatic enzyme inducers are taken concurrently with Norpace or Norpace CR, lower plasma levels of disopyramide may occur. Monitoring of disopyramide plasma levels is recommended in such concurrent use to avoid ineffective therapy. Other antiarrhythmic drugs (e.g., quinidine, procainamide, lidocaine, propranolol) have occasionally been used concurrently with Norpace. Excessive widening of the QRS complex and/or prolongation of the Q-T interval may occur in these situations (see Warnings). In healthy subjects, no significant drug-drug interaction was observed when Norpace was coadministered with either propranolol or diazepam. Concomitant administration of Norpace and quinidine resulted in slight increases in plasma disopyramide levels and slight decreases in plasma quinidine levels. Norpace does not increase serum digoxin levels.
Until data on possible interactions between verapamil and disopyramide phosphate are obtained, disopyramide should not be administered within 48 hours before or 24 hours after verapamil administration.
Although potent inhibitors of cytochrome P450 3A4 (e.g., ketoconazole) have not been studied clinically, in vitro studies have shown that erythromycin and oleandomycin inhibit the metabolism of disopyramide. Cases of life-threatening interactions have been reported for disopyramide when given with clarithromycin and erythromycin indicating that coadministration of disopyramide with inhibitors of cytochrome 3A4 could result in potentially fatal interaction.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Eighteen months of Norpace administration to rats, at oral doses up to 400 mg/kg/day (about 30 times the usual daily human dose of 600 mg/day, assuming a patient weight of at least 50 kg), revealed no evidence of carcinogenic potential. An evaluation of mutagenic potential by Ames test was negative. Norpace, at doses up to 250 mg/kg/day, did not adversely affect fertility of rats.
Pregnancy
Teratogenic Effects
Norpace was associated with decreased numbers of implantation sites and decreased growth and survival of pups when administered to pregnant rats at 250 mg/kg/day (20 or more times the usual daily human dose of 12 mg/kg, assuming a patient weight of at least 50 kg), a level at which weight gain and food consumption of dams were also reduced. Increased resorption rates were reported in rabbits at 60 mg/kg/day (5 or more times the usual daily human dose). Effects on implantation, pup growth, and survival were not evaluated in rabbits. There are no adequate and well-controlled studies in pregnant women. Norpace or Norpace CR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects: Norpace has been reported to stimulate contractions of the pregnant uterus. Disopyramide has been found in human fetal blood.
Labor and Delivery
It is not known whether the use of Norpace or Norpace CR during labor or delivery has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for forceps delivery or other obstetric intervention.
Nursing Mothers
Studies in rats have shown that the concentration of disopyramide and its metabolites is between one and three times greater in milk than it is in plasma. Following oral administration, disopyramide has been detected in human milk at a concentration not exceeding that in plasma. Because of the potential for serious adverse reactions in nursing infants from Norpace or Norpace CR, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established (see Dosage and Administration).
Geriatric Use
Clinical studies of Norpace/Norpace CR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Because of its anticholinergic activity, disopyramide phosphate should not be used in patients with glaucoma, urinary retention, or benign prostatic hypertrophy (medical conditions commonly associated with the elderly) unless adequate overriding measures are taken (see Warnings: Anticholinergic Activity). In the event of increased anticholinergic side effects, plasma levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see Precautions: Renal Impairment and Dosage and Administration).
-
ADVERSE REACTIONS
The adverse reactions which were reported in Norpace clinical trials encompass observations in 1,500 patients, including 90 patients studied for at least 4 years. The most serious adverse reactions are hypotension and congestive heart failure. The most common adverse reactions, which are dose dependent, are associated with the anticholinergic properties of the drug. These may be transitory, but may be persistent or can be severe. Urinary retention is the most serious anticholinergic effect.
The following reactions were reported in 10% to 40% of patients:
Anticholinergic: dry mouth (32%), urinary hesitancy (14%), constipation (11%)
The following reactions were reported in 3% to 9% of patients:
Anticholinergic: blurred vision, dry nose/eyes/throat
Genitourinary: urinary retention, urinary frequency and urgency
Gastrointestinal: nausea, pain/bloating/gas
General: dizziness, general fatigue/muscle weakness, headache, malaise, aches/pains
The following reactions were reported in 1% to 3% of patients:
Genitourinary: impotence
Cardiovascular: hypotension with or without congestive heart failure, increased congestive heart failure (see Warnings), cardiac conduction disturbances (see Warnings), edema/weight gain, shortness of breath, syncope, chest pain
Gastrointestinal: anorexia, diarrhea, vomiting
Dermatologic: generalized rash/dermatoses, itching
Central nervous system: nervousness
Other: hypokalemia, elevated cholesterol/triglycerides
The following reactions were reported in less than 1%:
Depression, insomnia, dysuria, numbness/tingling, elevated liver enzymes, AV block, elevated BUN, elevated creatinine, decreased hemoglobin/hematocrit
Hypoglycemia has been reported in association with Norpace administration (see Warnings).
Infrequent occurrences of reversible cholestatic jaundice, fever, and respiratory difficulty have been reported in association with disopyramide therapy, as have rare instances of thrombocytopenia, reversible agranulocytosis, and gynecomastia. Some cases of LE (lupus erythematosus) symptoms have been reported; most cases occurred in patients who had been switched to disopyramide from procainamide following the development of LE symptoms. Rarely, acute psychosis has been reported following Norpace therapy, with prompt return to normal mental status when therapy was stopped. The physician should be aware of these possible reactions and should discontinue Norpace or Norpace CR therapy promptly if they occur.
-
OVERDOSAGE
Symptoms
Deliberate or accidental overdosage of oral disopyramide may be followed by apnea, loss of consciousness, cardiac arrhythmias, and loss of spontaneous respiration. Death has occurred following overdosage.
Toxic plasma levels of disopyramide produce excessive widening of the QRS complex and Q-T interval, worsening of congestive heart failure, hypotension, varying kinds and degrees of conduction disturbance, bradycardia, and finally asystole. Obvious anticholinergic effects are also observed.
The approximate oral LD50 of disopyramide phosphate is 580 and 700 mg/kg for rats and mice, respectively.
Treatment
Experience indicates that prompt and vigorous treatment of overdosage is necessary, even in the absence of symptoms. Such treatment may be life-saving. No specific antidote for disopyramide phosphate has been identified. Treatment should be symptomatic and may include induction of emesis or gastric lavage, administration of a cathartic followed by activated charcoal by mouth or stomach tube, intravenous administration of isoproterenol and dopamine, insertion of an intra-aortic balloon for counterpulsation, and mechanically assisted ventilation. Hemodialysis or, preferably, hemoperfusion with charcoal may be employed to lower serum concentration of the drug.
The electrocardiogram should be monitored, and supportive therapy with cardiac glycosides and diuretics should be given as required.
If progressive AV block should develop, endocardial pacing should be implemented. In case of any impaired renal function, measures to increase the glomerular filtration rate may reduce the toxicity (disopyramide is excreted primarily by the kidney).
The anticholinergic effects can be reversed with neostigmine at the discretion of the physician.
Altering the urinary pH in humans does not affect the plasma half-life or the amount of disopyramide excreted in the urine.
-
DOSAGE AND ADMINISTRATION
The dosage of Norpace or Norpace CR must be individualized for each patient on the basis of response and tolerance. The usual adult dosage of Norpace or Norpace CR is 400 to 800 mg per day given in divided doses. The recommended dosage for most adults is 600 mg/day given in divided doses (either 150 mg every 6 hours for immediate-release Norpace or 300 mg every 12 hours for Norpace CR). For patients whose body weight is less than 110 pounds (50 kg), the recommended dosage is 400 mg/day given in divided doses (either 100 mg every 6 hours for immediate-release Norpace or 200 mg every 12 hours for Norpace CR). In the event of increased anticholinergic side effects, plasma levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval.
For patients with cardiomyopathy or possible cardiac decompensation, a loading dose, as discussed below, should not be given, and initial dosage should be limited to 100 mg of immediate-release Norpace every 6 to 8 hours. Subsequent dosage adjustments should be made gradually, with close monitoring for the possible development of hypotension and/or congestive heart failure (see Warnings).
For patients with moderate renal insufficiency (creatinine clearance greater than 40 ml/min) or hepatic insufficiency, the recommended dosage is 400 mg/day given in divided doses (either 100 mg every 6 hours for immediate-release Norpace or 200 mg every 12 hours for Norpace CR).
For patients with severe renal insufficiency (Ccr 40 ml/min or less), the recommended dosage regimen of immediate-release Norpace is 100 mg at intervals shown in the table below, with or without an initial loading dose of 150 mg.
IMMEDIATE-RELEASE NORPACE DOSAGE INTERVAL FOR PATIENTS WITH RENAL INSUFFICIENCY Creatinine Clearance
(ml/min)40–30
30–15
Less than 15
Approximate Maintenance-dosing interval
q 8 hr
q 12 hr
Q 24 hr
The above dosing schedules are for Norpace immediate-release capsules; Norpace CR is not recommended for patients with severe renal insufficiency.
For patients in whom rapid control of ventricular arrhythmia is essential, an initial loading dose of 300 mg of immediate-release Norpace (200 mg for patients whose body weight is less than 110 pounds) is recommended, followed by the appropriate maintenance dosage. Therapeutic effects are usually attained 30 minutes to 3 hours after administration of a 300-mg loading dose. If there is no response or evidence of toxicity within 6 hours of the loading dose, 200 mg of immediate-release Norpace every 6 hours may be prescribed instead of the usual 150 mg. If there is no response to this dosage within 48 hours, either Norpace should then be discontinued or the physician should consider hospitalizing the patient for careful monitoring while subsequent immediate-release Norpace doses of 250 mg or 300 mg every 6 hours are given. A limited number of patients with severe refractory ventricular tachycardia have tolerated daily doses of Norpace up to 1600 mg per day (400 mg every 6 hours), resulting in disopyramide plasma levels up to 9 mcg/ml. If such treatment is warranted, it is essential that patients be hospitalized for close evaluation and continuous monitoring.
Norpace CR should not be used initially if rapid establishment of disopyramide plasma levels is desired.
Transferring to Norpace or Norpace CR.
The following dosage schedule based on theoretical considerations rather than experimental data is suggested for transferring patients with normal renal function from either quinidine sulfate or procainamide therapy (Type 1 antiarrhythmic agents) to Norpace or Norpace CR therapy:
Norpace or Norpace CR should be started using the regular maintenance schedule without a loading dose 6 to 12 hours after the last dose of quinidine sulfate or 3 to 6 hours after the last dose of procainamide.
In patients in whom withdrawal of quinidine sulfate or procainamide is likely to produce life-threatening arrhythmias, the physician should consider hospitalization of the patient. When transferring a patient from immediate-release Norpace to Norpace CR, the maintenance schedule of Norpace CR may be started 6 hours after the last dose of immediate-release Norpace.
Pediatric Dosage
Controlled clinical studies have not been conducted in pediatric patients; however, the following suggested dosage table is based on published clinical experience.
Total daily dosage should be divided and equal doses administered orally every 6 hours or at intervals according to individual patient needs. Disopyramide plasma levels and therapeutic response must be monitored closely. Patients should be hospitalized during the initial treatment period, and dose titration should start at the lower end of the ranges provided below.
SUGGESTED TOTAL DAILY DOSAGE* Age
(years)Disopyramide
(mg/kg body weight/day)- *
- Dosage is expressed in milligrams of disopyramide base. Since Norpace (disopyramide phosphate) 100-mg capsules contain 100 mg of disopyramide base, the pharmacist can readily prepare a 1-mg/ml to 10-mg/ml liquid suspension by adding the entire contents of Norpace capsules to cherry syrup. (Prepare cherry syrup as follows: cherry juice, 475 ml; sucrose 800 g; alcohol, 20 ml; purified water, a sufficient quantity to make 1000 ml.) The resulting suspension, when refrigerated, is stable for one month and should be thoroughly shaken before the measurement of each dose. The suspension should be dispensed in an amber glass bottle with a child-resistant closure.
Under 1
10 to 30
1 to 4
10 to 20
4 to 12
10 to 15
12 to 18
6 to 15
Norpace CR capsules should not be used to prepare the above suspension.
-
HOW SUPPLIED
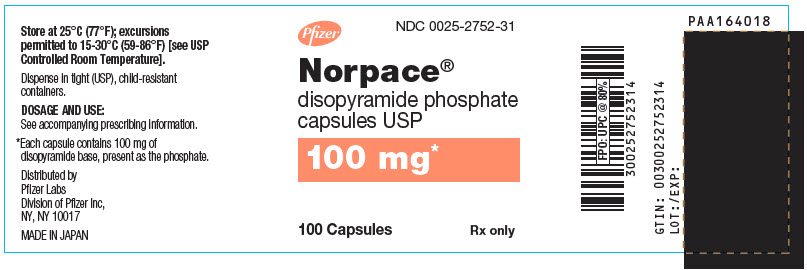
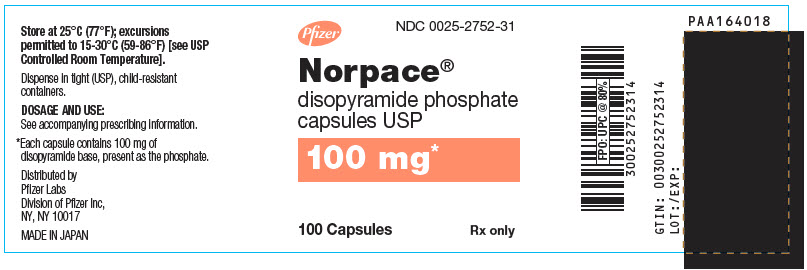
Norpace (disopyramide phosphate) is supplied in hard gelatin capsules containing either 100 mg or 150 mg of disopyramide base, present as the phosphate.
Norpace 100-mg capsules are white and orange, with markings SEARLE, 2752, NORPACE, and 100 MG.
NDC Number Size 0025-2752-31
bottle of 100
Norpace 150-mg capsules are brown and orange, with markings SEARLE, 2762, NORPACE, and 150 MG.
NDC Number Size 0025-2762-31
bottle of 100
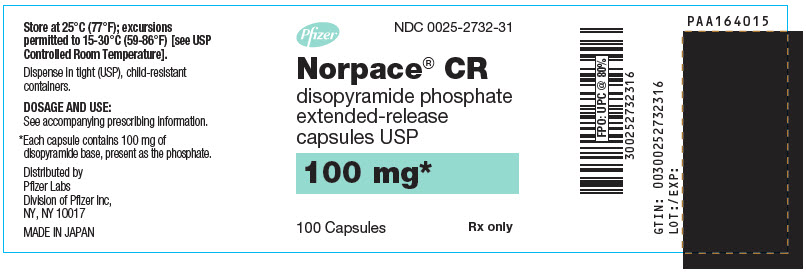
Norpace CR (disopyramide phosphate) Controlled-Release is supplied as specially prepared controlled-release beads in hard gelatin capsules containing either 100 mg or 150 mg of disopyramide base, present as the phosphate.
Norpace CR 100-mg capsules are white and light green, with markings SEARLE, 2732, NORPACE CR, and 100 mg.
NDC Number Size 0025-2732-31
bottle of 100
Norpace CR 150-mg capsules are brown and light green, with markings SEARLE, 2742, NORPACE CR, and 150 mg.
NDC Number Size 0025-2742-31
bottle of 100
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label
- PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
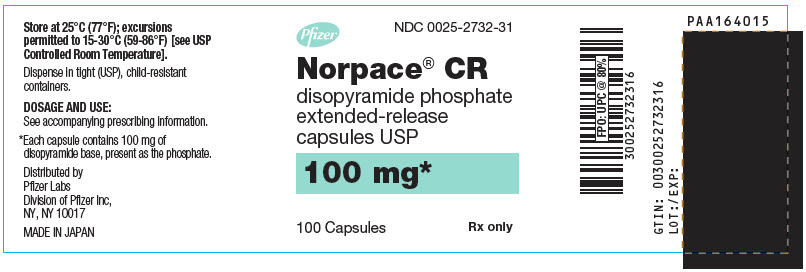
- PRINCIPAL DISPLAY PANEL - 100 mg Capsule Bottle Label - NDC 0025-2732-31
- PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label - NDC 0025-2742-31
-
INGREDIENTS AND APPEARANCE
NORPACE
disopyramide phosphate capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0025-2752 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DISOPYRAMIDE PHOSPHATE (UNII: N6BOM1935W) (DISOPYRAMIDE - UNII:GFO928U8MQ) DISOPYRAMIDE 100 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE, ORANGE Score no score Shape CAPSULE Size 19mm Flavor Imprint Code SEARLE;2752;NORPACE;100;MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0025-2752-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/1977 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017447 09/01/1977 NORPACE
disopyramide phosphate capsule, gelatin coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0025-2762 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DISOPYRAMIDE PHOSPHATE (UNII: N6BOM1935W) (DISOPYRAMIDE - UNII:GFO928U8MQ) DISOPYRAMIDE 150 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color BROWN, ORANGE Score no score Shape CAPSULE Size 19mm Flavor Imprint Code SEARLE;2762;NORPACE;150;MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0025-2762-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 09/01/1977 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017447 09/01/1977 NORPACE CR
disopyramide phosphate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0025-2732 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DISOPYRAMIDE PHOSPHATE (UNII: N6BOM1935W) (DISOPYRAMIDE - UNII:GFO928U8MQ) DISOPYRAMIDE 100 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) Product Characteristics Color WHITE, GREEN (Light Green) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code SEARLE;2732;NORPACE;CR;100;MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0025-2732-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018655 07/20/1982 NORPACE CR
disopyramide phosphate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0025-2742 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DISOPYRAMIDE PHOSPHATE (UNII: N6BOM1935W) (DISOPYRAMIDE - UNII:GFO928U8MQ) DISOPYRAMIDE 150 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) SUCROSE (UNII: C151H8M554) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color BROWN, GREEN (Light Green) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code SEARLE;2742;NORPACE;CR;150;MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0025-2742-31 100 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/1982 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA018655 07/20/1982 Labeler - Pfizer Laboratories Div Pfizer Inc (134489525)