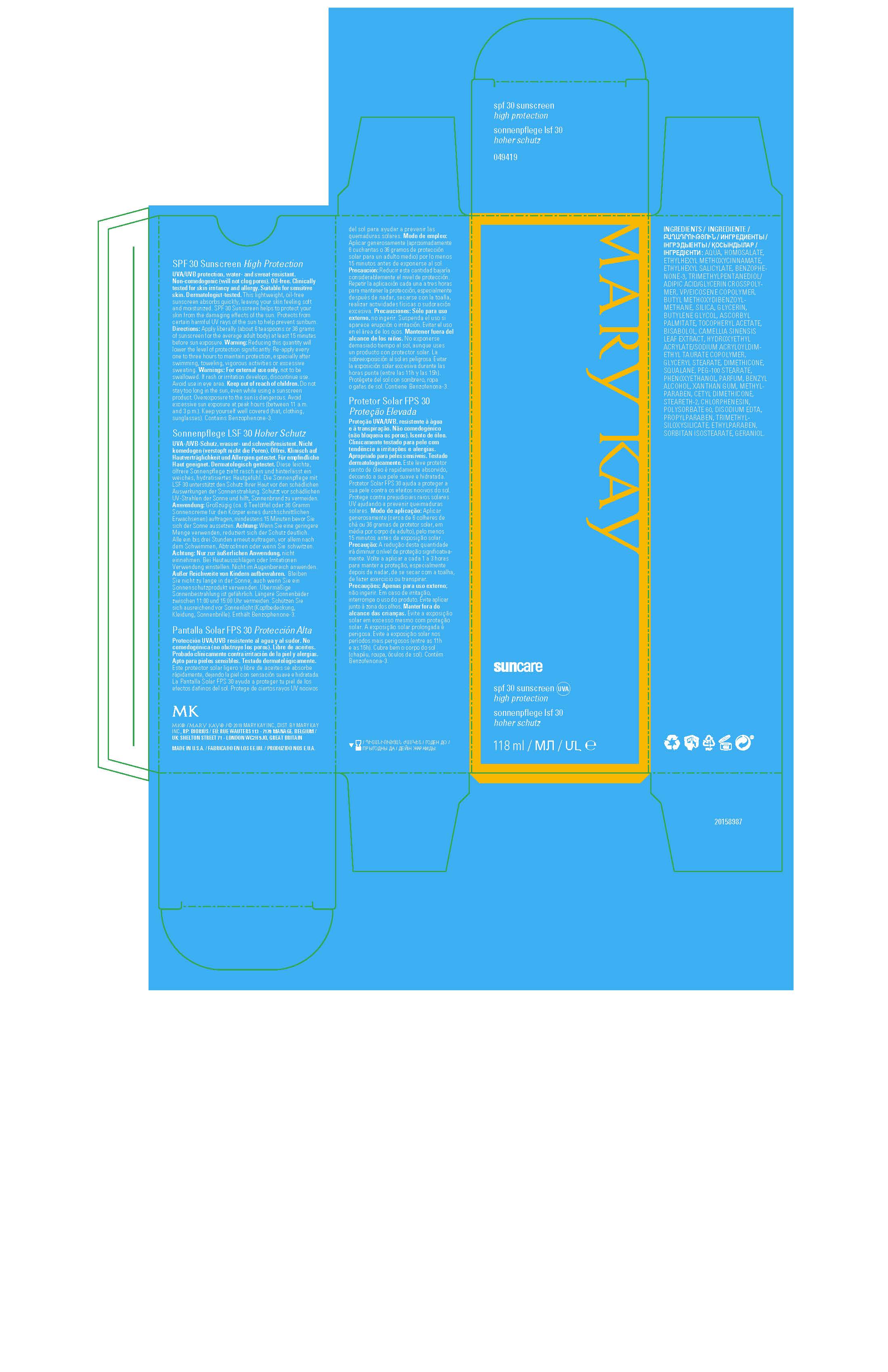
Label: MARY KAY SUNCARE SPF 30 SUNSCREEN- avobenzone, homosalate, octinoxate, octisalate, oxybenzone lotion
- NDC Code(s): 51531-9419-4
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
Warnings:
For external use only, not to be swallowed.
If rash or irritation develops, discontinue use.
Avoid use in eye area.
Keep out of reach of children.
Do not stay too long in the sun, even while using a sunscreen product.
Overexposure to the sun is dangerous.
Avoid excessive sun exposure at peak hours (between 11 a.m. and 3 p.m.).
Keep yourself well covered (hat, clothing, sunglasses).
Contains Benzophenone-3.
-
Directions:
Apply liberally (about 6 teaspoons or 36 grams of sunscreen for the average adult body) at least 15 minutes before sun exposure.
Warning: Reducing this quantity will lower the level of protection significantly.
Re-apply every one to three hours to maintain protection, especially after swimming, toweling, vigorous activities or excessive sweating.
-
Ingredients:
AQUA, HOMOSALATE, ETHYLHEXYL METHOXYCINNAMATE, ETHYLHEXYL SALICYLATE, BENZOPHENONE-3, TRIMETHYLPENTANEDIOL/
ADIPIC ACID/GLYCERIN CROSSPOLYMER, VP/EICOSENE COPOLYMER, BUTYL METHOXYDIBENZOYLMETHANE, SILICA, GLYCERIN,
BUTYLENE GLYCOL, ASCORBYL PALMITATE, TOCOPHERYL ACETATE, BISABOLOL, CAMELLIA SINENSIS LEAF EXTRACT, HYDROXYETHYL
ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER, GLYCERYL STEARATE, DIMETHICONE, SQUALANE, PEG-100 STEARATE,
PHENOXYETHANOL, PARFUM, BENZYL ALCOHOL, XANTHAN GUM, METHYLPARABEN, CETYL DIMETHICONE, STEARETH-2, CHLORPHENESIN,
POLYSORBATE 60, DISODIUM EDTA, PROPYLPARABEN, TRIMETHYLSILOXYSILICATE, ETHYLPARABEN, SORBITAN ISOSTEARATE, GERANIOL. - Principal Display Panel - 118 mL carton
-
INGREDIENTS AND APPEARANCE
MARY KAY SUNCARE SPF 30 SUNSCREEN
avobenzone, homosalate, octinoxate, octisalate, oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-9419 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) TRIMETHYLPENTANEDIOL/ADIPIC ACID/GLYCERIN CROSSPOLYMER (25000 MPA.S) (UNII: 587WKM3S9Q) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIMETHICONE (UNII: 92RU3N3Y1O) SQUALANE (UNII: GW89575KF9) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZYL ALCOHOL (UNII: LKG8494WBH) XANTHAN GUM (UNII: TTV12P4NEE) METHYLPARABEN (UNII: A2I8C7HI9T) LEVOMENOL (UNII: 24WE03BX2T) STEARETH-2 (UNII: V56DFE46J5) CHLORPHENESIN (UNII: I670DAL4SZ) POLYSORBATE 60 (UNII: CAL22UVI4M) ASCORBYL PALMITATE (UNII: QN83US2B0N) EDETATE DISODIUM (UNII: 7FLD91C86K) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) ETHYLPARABEN (UNII: 14255EXE39) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) GREEN TEA LEAF (UNII: W2ZU1RY8B0) GERANIOL (UNII: L837108USY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-9419-4 1 in 1 CARTON 05/07/2013 1 118 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 05/07/2013 07/12/2025 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-9419)