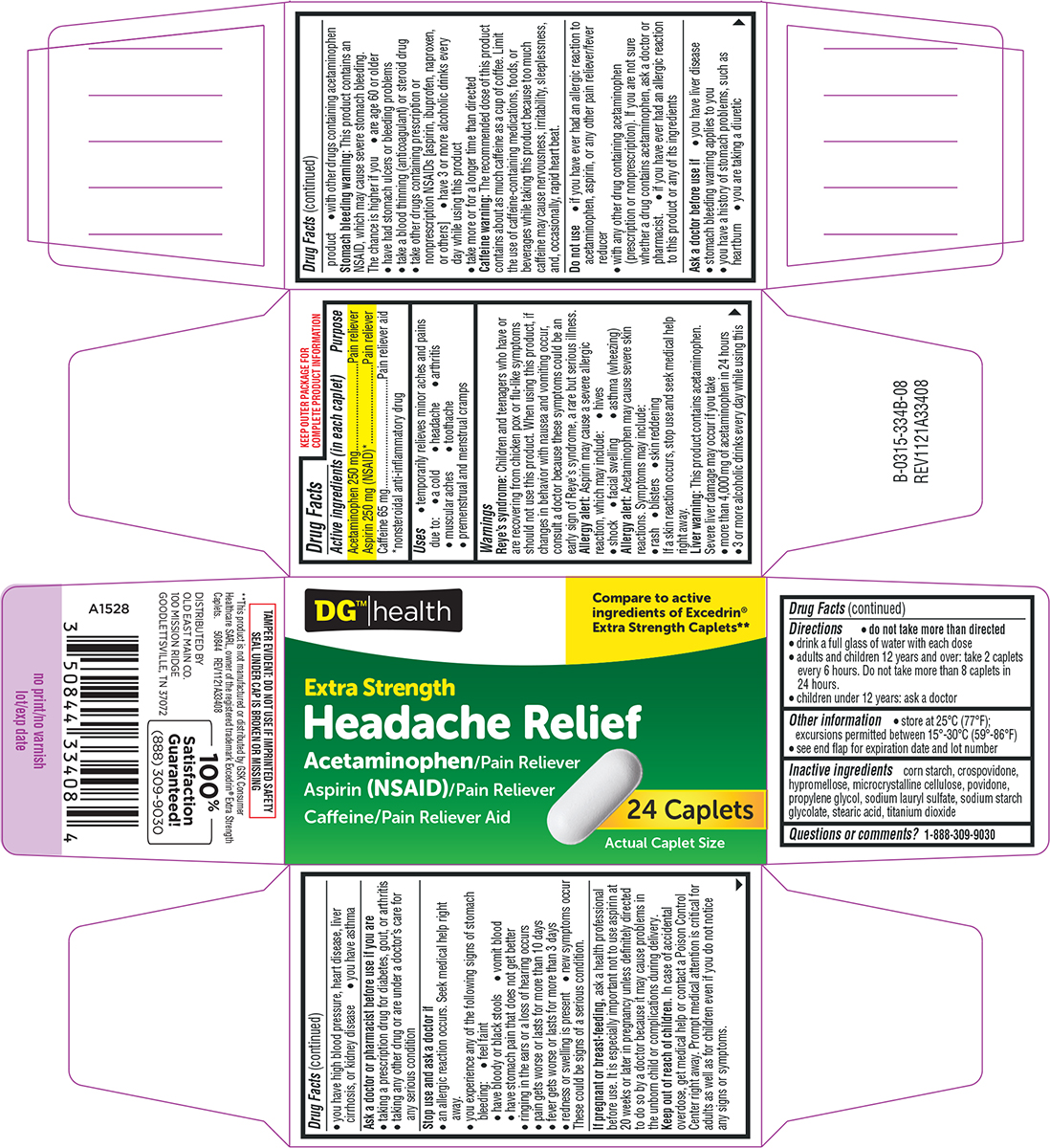
Label: HEADACHE RELIEF- acetaminophen, aspirin, caffeine tablet, film coated
- NDC Code(s): 55910-345-08, 55910-345-12
- Packager: DOLGENCORP, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 3, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredients (in each caplet)
Acetaminophen 250 mg - Aspirin 250 mg (NSAID*) Caffeine 65 mg - *nonsteroidal anti-inflammatory drug
-
Purpose
Pain reliever - Pain reliever - Pain reliever aid
-
Uses
temporarily relieves minor aches and pains due to: headache - arthritis - a cold - muscular aches - toothache - premenstrual and menstrual cramps
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea ...
-
Directions
do not take more than directed - drink a full glass of water with each dose - adults and children 12 years and over: take 2 caplets every 6 hours. Do not take more than 8 caplets in 24 ...
-
Other information
store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) see end flap for expiration date and lot number
-
Inactive ingredients
corn starch, crospovidone, hypromellose, microcrystalline cellulose, povidone, propylene glycol, sodium lauryl sulfate, sodium starch glycolate, stearic acid, titanium dioxide
-
Questions or comments?
1-888-309-9030
-
Principal display panel
DG® | health - Compare to - active ingredients of - Excedrin® Extra Strength - Headache** Extra Strength - Headache Relief - Acetaminophen, Aspirin (NSAID) and Caffeine - Pain Reliever / Pain ...
-
INGREDIENTS AND APPEARANCEProduct Information