Label: REGADENOSON injection
- NDC Code(s): 67457-994-05
- Packager: Mylan Institutional LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REGADENOSON INJECTION safely and effectively. See full prescribing information for REGADENOSON INJECTION.
REGADENOSON injection, for intravenous use
Initial U.S. Approval: 2008INDICATIONS AND USAGE
Regadenoson injection is a pharmacologic stress agent indicated for radionuclide myocardial perfusion imaging (MPI) in patients unable to undergo adequate exercise stress (1).
DOSAGE AND ADMINISTRATION
- •
- The recommended dose of regadenoson injection is 5 mL (0.4 mg regadenoson) administered as an intravenous injection within 10 seconds; followed immediately by saline flush and radiopharmaceutical (2).
DOSAGE FORMS AND STRENGTHS
- •
- Injection: Single-dose pre-filled syringe: 0.4 mg/5 mL (0.08 mg/mL) (3).
CONTRAINDICATIONS
Do not administer regadenoson to patients with:
- •
- Second- or third-degree AV block, or
- •
- sinus node dysfunction
unless the patients have a functioning artificial pacemaker (4).
WARNINGS AND PRECAUTIONS
- •
- Myocardial Ischemia. Fatal cardiac events have occurred. Avoid use in patients with symptoms or signs of acute myocardial ischemia, for example unstable angina or cardiovascular instability, who may be at greater risk. Cardiac resuscitation equipment and trained staff should be available before administration (5.1).
- •
- Sinoatrial (SA) and Atrioventricular (AV) Nodal Block. Adenosine receptor agonists, including regadenoson, can depress the SA and AV nodes and may cause first-, second- or third-degree AV block, or sinus bradycardia (5.2).
- •
- Atrial Fibrillation/Atrial Flutter. New-onset or recurrent atrial fibrillation with rapid ventricular response and atrial flutter have been reported (5.3).
- •
- Hypersensitivity, including anaphylaxis, angioedema, cardiac or respiratory arrest, respiratory distress, decreased oxygen saturation, hypotension, throat tightness, urticaria, and rashes have occurred. Have personnel and resuscitative equipment immediately available (5.4).
- •
- Hypotension. Adenosine receptor agonists, including regadenoson, induce vasodilation and hypotension. The risk of serious hypotension may be higher in patients with autonomic dysfunction, stenotic valvular heart disease, pericarditis or pericardial effusions, stenotic carotid artery disease with cerebrovascular insufficiency, or hypovolemia (5.5).
- •
- Hypertension. Adenosine receptor agonists, including regadenoson, may induce clinically significant increases in blood pressure particularly in patients with a history of hypertension and when the MPI includes low level exercise (5.6).
- •
- Bronchoconstriction. Adenosine receptor agonists, including regadenoson, may induce dyspnea, bronchoconstriction and respiratory compromise in patients with chronic obstructive pulmonary disease (COPD) or asthma. Resuscitative measures should be available (5.7).
- •
- Seizure. Regadenoson may lower the seizure threshold. New onset or recurrence of convulsive seizures has occurred. Some seizures are prolonged and require urgent anticonvulsive management. Methylxanthine use is not recommended in patients who experience a seizure in association with regadenoson (5.8).
- •
- Cerebrovascular Accident (Stroke). Hemorrhagic and ischemic cerebrovascular accidents have occurred (5.9).
ADVERSE REACTIONS
The most common (incidence ≥ 5%) adverse reactions to regadenoson are dyspnea, headache, flushing, chest discomfort, dizziness, angina pectoris, chest pain, and nausea (6).
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Methylxanthines, e.g., caffeine, aminophylline and theophylline, interfere with the activity of regadenoson (7.1, 12.2).
- •
- Aminophylline may be used to attenuate severe and/or persistent adverse reactions to regadenoson (7.1, 10).
- •
- Dipyridamole may increase the activity of regadenoson. When possible, withhold dipyridamole for at least two days prior to regadenoson administration (7.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia
5.2 Sinoatrial and Atrioventricular Nodal Block
5.3 Atrial Fibrillation/Atrial Flutter
5.4 Hypersensitivity, Including Anaphylaxis
5.5 Hypotension
5.6 Hypertension
5.7 Bronchoconstriction
5.8 Seizure
5.9 Cerebrovascular Accident (Stroke)
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Regadenoson
7.2 Effect of Regadenoson on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended dose of regadenoson injection is 5 mL (0.4 mg regadenoson) administered as an intravenous injection within 10 seconds.
- •
- Patients should be instructed to avoid consumption of any products containing methylxanthines, including caffeinated coffee, tea or other caffeinated beverages, caffeine-containing drug products, aminophylline and theophylline for at least 12 hours before a scheduled radionuclide MPI [see Drug Interactions (7.1) and Clinical Pharmacology (12.2)].
- •
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not administer regadenoson injection if it contains particulate matter or is discolored.
- •
- Administer regadenoson as an intravenous injection within 10 seconds into a peripheral vein using a 22 gauge or larger catheter or needle.
- •
- Administer a 5 mL saline flush immediately after the injection of regadenoson injection.
- •
- Administer the radionuclide myocardial perfusion imaging agent 10 to 20 seconds after the saline flush. The radionuclide may be injected directly into the same catheter as regadenoson injection.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Do not administer regadenoson to patients with:
- •
- Second- or third-degree AV block, or
- •
- sinus node dysfunction
unless these patients have a functioning artificial pacemaker [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia
Fatal and nonfatal myocardial infarction (MI), ventricular arrhythmias, and cardiac arrest have occurred following regadenoson injection. Avoid use in patients with symptoms or signs of acute myocardial ischemia, for example unstable angina or cardiovascular instability; these patients may be at greater risk of serious cardiovascular reactions to regadenoson. Cardiac resuscitation equipment and trained staff should be available before administering regadenoson. Adhere to the recommended duration of injection [see Dosage and Administration (2)]. As noted in an animal study, longer injection times may increase the duration and magnitude of increase in coronary blood flow [see Clinical Pharmacology (12.2)]. If serious reactions to regadenoson occur, consider the use of aminophylline, an adenosine antagonist, to shorten the duration of increased coronary blood flow induced by regadenoson [see Overdosage (10)].
5.2 Sinoatrial and Atrioventricular Nodal Block
Adenosine receptor agonists, including regadenoson, can depress the SA and AV nodes and may cause first-, second- or third-degree AV block, or sinus bradycardia requiring intervention. In clinical trials first-degree AV block (PR prolongation > 220 msec) developed in 3% of patients within 2 hours of regadenoson administration; transient second-degree AV block with one dropped beat was observed in one patient receiving regadenoson. In post-marketing experience, third-degree heart block and asystole within minutes of regadenoson administration have occurred [see Adverse Reactions (6.2)].
5.3 Atrial Fibrillation/Atrial Flutter
New-onset or recurrent atrial fibrillation with rapid ventricular response and atrial flutter have been reported following regadenoson injection [see Adverse Reactions (6.2)].
5.4 Hypersensitivity, Including Anaphylaxis
Anaphylaxis, angioedema, cardiac or respiratory arrest, respiratory distress, decreased oxygen saturation, hypotension, throat tightness, urticaria and rashes have occurred. In clinical trials, hypersensitivity reactions were reported in fewer than 1 percent of patients [see Adverse Reactions (6.1)]. Have personnel and resuscitative equipment immediately available.
5.5 Hypotension
Adenosine receptor agonists, including regadenoson, induce arterial vasodilation and hypotension. In clinical trials, decreased systolic blood pressure (> 35 mm Hg) was observed in 7% of patients and decreased diastolic blood pressure (> 25 mm Hg) was observed in 4% of patients within 45 minutes of regadenoson administration. The risk of serious hypotension may be higher in patients with autonomic dysfunction, hypovolemia, left main coronary artery stenosis, stenotic valvular heart disease, pericarditis or pericardial effusions, or stenotic carotid artery disease with cerebrovascular insufficiency. In post-marketing experience, syncope, transient ischemic attacks and seizures have been observed [see Adverse Reactions (6.2)].
5.6 Hypertension
Administration of adenosine receptor agonists, including regadenoson, may result in clinically significant increases in blood pressure in some patients. Among patients who experienced an increase in blood pressure in clinical trials, the increase was observed within minutes of regadenoson administration. Most increases resolved within 10 to 15 minutes, but in some cases, increases were observed at 45 minutes following administration [see Clinical Pharmacology (12.2)]. In post-marketing experience, cases of potentially clinically significant hypertension have been reported, particularly with underlying hypertension and when low-level exercise was included in the MPI [see Adverse Reactions (6.2)].
5.7 Bronchoconstriction
Adenosine receptor agonists, including regadenoson, may cause dyspnea, bronchoconstriction, and respiratory compromise. Appropriate bronchodilator therapy and resuscitative measures should be available prior to and following regadenoson administration [see Adverse Reactions (6.1), Clinical Pharmacology (12.2), Overdosage (10) and Patient Counseling Information (17)].
5.8 Seizure
Regadenoson may lower the seizure threshold; obtain a seizure history. New-onset or recurrence of convulsive seizures has occurred following regadenoson injection. Some seizures are prolonged and require emergent anticonvulsive management. Aminophylline may increase the risk of seizures associated with regadenoson injection. Methylxanthine use is not recommended in patients who experience a seizure in association with regadenoson administration.
5.9 Cerebrovascular Accident (Stroke)
Hemorrhagic and ischemic cerebrovascular accidents have occurred. Hemodynamic effects of regadenoson including hypotension or hypertension may be associated with these adverse reactions [see Warnings and Precautions (5.5) and (5.6)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling.
- •
- Myocardial Ischemia [see Warnings and Precautions (5.1)]
- •
- Sinoatrial and Atrioventricular Nodal Block [see Warnings and Precautions (5.2)]
- •
- Atrial Fibrillation/Atrial Flutter [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity, Including Anaphylaxis [see Warnings and Precautions (5.4)]
- •
- Hypotension [see Warnings and Precautions (5.5)]
- •
- Hypertension [see Warnings and Precautions (5.6)]
- •
- Bronchoconstriction [see Warnings and Precautions (5.7)]
- •
- Seizure [see Warnings and Precautions (5.8)]
- •
- Cerebrovascular Accident (Stroke) [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
During clinical development, 1,651 patients were exposed to regadenoson, with most receiving 0.4 mg as a rapid (≤ 10 seconds) intravenous injection. Most of these patients received regadenoson in two clinical studies that enrolled patients who had no history of bronchospastic lung disease as well as no history of a cardiac conduction block of greater than first-degree AV block, except for patients with functioning artificial pacemakers. In these studies (Studies 1 and 2), 2,015 patients underwent myocardial perfusion imaging after administration of regadenoson (N = 1,337) or adenosine (N = 678). The population was 26 to 93 years of age (median 66 years), 70% male and primarily Caucasian (76% Caucasian, 7% African American, 9% Hispanic, 5% Asian). Table 1 shows the most frequently reported adverse reactions.
Overall, any adverse reaction occurred at similar rates between the study groups (80% for the regadenoson group and 83% for the adenosine group). Aminophylline was used to treat the reactions in 3% of patients in the regadenoson group and 2% of patients in the adenosine group. Most adverse reactions began soon after dosing, and generally resolved within approximately 15 minutes, except for headache which resolved in most patients within 30 minutes.
Table 1 Adverse Reactions in Studies 1 and 2 Pooled (Frequency ≥ 5%) Regadenoson
N = 1,337
Adenosine
N = 678
Dyspnea
28%
26%
Headache
26%
17%
Flushing
16%
25%
Chest Discomfort
13%
18%
Angina Pectoris or ST Segment Depression
12%
18%
Dizziness
8%
7%
Chest Pain
7%
10%
Nausea
6%
6%
Abdominal Discomfort
5%
2%
Dysgeusia
5%
7%
Feeling Hot
5%
8%
ECG Abnormalities
The frequency of rhythm or conduction abnormalities following regadenoson or adenosine is shown in Table 2 [see Warnings and Precautions (5.2)].
Table 2 Rhythm or Conduction Abnormalities* in Studies 1 and 2 Regadenoson
N / N evaluable (%)
Adenosine
N / N evaluable (%)
Rhythm or conduction abnormalities†
332/1275 (26%)
192/645 (30%)
Rhythm abnormalities
260/1275 (20%)
131/645 (20%)
PACs
86/1274 (7%)
57/645 (9%)
PVCs
179/1274 (14%)
79/645 (12%)
First-degree AV block (PR prolongation > 220 msec)
34/1209 (3%)
43/618 (7%)
Second-degree AV block
1/1209 (0.1%)
9/618 (1%)
AV conduction abnormalities (other than AV blocks)
1/1209 (0.1%)
0/618 (0%)
Ventricular conduction abnormalities
64/1152 (6%)
31/581 (5%)
Respiratory Abnormalities
In a randomized, placebo-controlled trial of 999 patients with asthma (n = 532) or stable chronic obstructive pulmonary disease (n = 467), the overall incidence of pre-specified respiratory adverse reactions was greater in the regadenoson group compared to the placebo group (p < 0.001). Most respiratory adverse reactions resolved without therapy; a few patients received aminophylline or a short-acting bronchodilator. No differences were observed between treatment arms in the reduction of >15% from baseline at two-hours in FEV1 (Table 3).
Table 3 Respiratory Adverse Effects* - *
- All patients continued the use of their respiratory medications as prescribed prior to administration of regadenoson.
- †
- Patients may have reported more than one type of adverse reaction. Adverse reactions were collected up to 24 hours following drug administration. Pre-specified respiratory adverse reactions included dyspnea, wheezing, obstructive airway disorder, dyspnea exertional, and tachypnea.
- ‡
- Change from baseline at 2 hours.
Asthma Cohort
Chronic Obstructive Pulmonary Disease
(COPD) Cohort
Regadenoson
(N=356)
Placebo
(N=176)
Regadenoson
(N=316)
Placebo
(N=151)
Overall Pre-specified
Respiratory Adverse Reaction†12.9%
2.3%
19.0%
4.0%
Dyspnea
10.7%
1.1%
18.0%
2.6%
Wheezing
3.1%
1.1%
0.9%
0.7%
FEV1 reduction >15%‡
1.1%
2.9%
4.2%
5.4%
Renal Impairment
In a randomized, placebo-controlled trial of 504 patients (regadenoson n=334 and placebo n=170) with a diagnosis or risk factors for coronary artery disease and NKFK/DOQI Stage III or IV renal impairment (defined as GFR 15 to 59 mL/min/1.73 m2), no serious adverse events were reported through the 24-hour follow-up period.
Inadequate Exercise Stress
In an open-label, multi-center trial evaluating regadenoson administration following inadequate exercise stress, 1,147 patients were randomized into one of two groups. Each group underwent two regadenoson stress myocardial perfusion imaging (MPI) procedures. Group 1 received regadenoson 3 minutes following inadequate exercise in the first regadenoson stress (MPI 1). Group 2 rested 1 hour after inadequate exercise to allow hemodynamics to return to baseline prior to receiving regadenoson (MPI 1). Both groups returned for a second stress MPI 1 to 14 days later and received regadenoson without exercise (MPI 2).
The most common adverse reactions are similar in type and incidence to those in Table 1 above for both Groups. The timing of the administration of regadenoson following inadequate exercise did not alter the common adverse reaction profile.
Table 4 shows a comparison of cardiac events of interest for the two groups [see Warnings and Precautions (5.1)]. The cardiac events were numerically higher in Group 1.
Table 4 Cardiac Events of Interest in Inadequate Exercise Stress Study - *
- A clinically significant cardiac event was defined as any of the following events found on the Holter ECG/12-lead ECG within one hour after regadenoson administration: ventricular arrhythmias (sustained ventricular tachycardia, ventricular fibrillation, Torsade de Pointes, ventricular flutter); ST-T depression (≥ 2 mm); ST-T elevation (≥ 1 mm); AV block (2:1 AV block, AV Mobitz I, AV Mobitz II, complete heart block); sinus arrest > 3 seconds in duration
Or
• a Treatment Emergent Adverse Event (TEAE) per the MedDRA SMQ (narrow Scope) for myocardial infarction
Or
• a TEAE preferred term (PT) of angina unstable within 24 hours of regadenoson administration.
Cardiac Event*
Group 1 / MPI 1
Regadenoson 3 minutes
following exercise
(N=575)
Group 2 / MPI 1
Regadenoson 1 hour
following exercise
(N=567)
17 (3.0%)
3 (0.5%)
Holter/12-Lead ECG Abnormality
ST-T Depression (≥ 2 mm)
13 (2.3%)
2 (0.4%)
ST-T Elevation (≥ 1 mm)
3 (0.5%)
1 (0.2%)
Acute coronary syndrome
1 (0.2%)
0
Myocardial infarction
1 (0.2%)
0
6.2 Post-Marketing Experience
The following adverse reactions have been reported from worldwide marketing experience with regadenoson. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular
Myocardial infarction, cardiac arrest, ventricular arrhythmias, supraventricular tachyarrhythmias including atrial fibrillation with rapid ventricular response (new-onset or recurrent), atrial flutter, heart block (including third-degree block), asystole, marked hypertension, symptomatic hypotension in association with transient ischemic attack, acute coronary syndrome (ACS), seizures and syncope [see Warnings and Precautions (5.1), (5.2), (5.3), (5.5), (5.6) and (5.8)] have been reported. Some events required intervention with fluids and/or aminophylline [see Overdosage (10)]. QTc prolongation shortly after regadenoson administration has been reported.
Central Nervous System
Tremor, seizure, transient ischemic attack, and cerebrovascular accident including intracranial hemorrhage [see Warnings and Precautions (5.8) and (5.9)].
Gastrointestinal
Abdominal pain, occasionally severe, has been reported a few minutes after regadenoson administration, in association with nausea, vomiting, or myalgias; administration of aminophylline, an adenosine antagonist, appeared to lessen the pain. Diarrhea and fecal incontinence have also been reported following regadenoson administration.
Hypersensitivity
Anaphylaxis, angioedema, cardiac or respiratory arrest, respiratory distress, decreased oxygen saturation, hypotension, throat tightness, urticaria, rashes have occurred and have required treatment including resuscitation [see Warnings and Precautions (5.4)].
Musculoskeletal
Musculoskeletal pain has occurred, typically 10 to 20 minutes after regadenoson administration; the pain was occasionally severe, localized in the arms and lower back and extended to the buttocks and lower legs bilaterally. Administration of aminophylline appeared to lessen the pain.
-
7 DRUG INTERACTIONS
No formal pharmacokinetic drug interaction studies have been conducted with regadenoson.
7.1 Effects of Other Drugs on Regadenoson
Methylxanthines (e.g., caffeine, aminophylline and theophylline) are non-specific adenosine receptor antagonists that interfere with the vasodilation activity of regadenoson [see Clinical Pharmacology (12.2) and Patient Counseling Information (17)]. Patients should avoid consumption of any products containing methylxanthines as well as any drugs containing theophylline or aminophylline for at least 12 hours before regadenoson administration. Aminophylline may be used to attenuate severe or persistent adverse reactions to regadenoson [see Overdosage (10)].
In clinical studies, regadenoson was administered to patients taking other cardioactive drugs (i.e., ß-blockers, calcium channel blockers, ACE inhibitors, nitrates, cardiac glycosides, and angiotensin receptor blockers) without reported adverse reactions or apparent effects on efficacy.
Dipyridamole may change the effects of regadenoson. When possible, withhold dipyridamole for at least two days prior to regadenoson administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on regadenoson use in pregnant women to inform a drug-associated risk. In animal reproduction studies, adverse developmental outcomes were observed with the administration of regadenoson to pregnant rats and rabbits during organogenesis only at doses that produced maternal toxicity (see Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
Reproductive studies in rats showed that regadenoson doses 10 and 20 times the maximum recommended human dose (MRHD) based on body surface area caused reduced fetal body weights and significant ossification delays in fore- and hind limb phalanges and metatarsals; maternal toxicity also occurred at these doses. Skeletal variations were increased in all treated groups. In rabbits, maternal toxicity occurred at regadenoson doses administered during organogenesis at 4 times the MRHD; however, there were no teratogenic effects in offspring at this dose. At higher doses, 12 and 20 times the MRHD, maternal toxicity occurred along with increased embryo-fetal loss and fetal malformations.
8.2 Lactation
Risk Summary
There is no information on the presence of regadenoson in human milk, the effects on the breastfed infant, or the effects on milk production. Because of the potential risk of serious cardiac reactions in the breastfed infant, advise the nursing mother to pump and discard breast milk for 10 hours after administration of regadenoson.
8.5 Geriatric Use
Of the 1,337 patients receiving regadenoson in Studies 1 and 2, 56% were 65 years of age and over and 24% were 75 years of age and over. Older patients (≥ 75 years of age) had a similar adverse event profile compared to younger patients (< 65 years of age), but had a higher incidence of hypotension (2% vs. ≤ 1%).
8.6 Renal Impairment
No dose adjustment is needed in patients with renal impairment including patients with end stage renal disease and/or dependent on dialysis [see Pharmacokinetics (12.3)].
-
10 OVERDOSAGE
Regadenoson overdosage may result in serious reactions [see Warnings and Precautions (5)]. In a study of healthy volunteers, symptoms of flushing, dizziness and increased heart rate were assessed as intolerable at regadenoson doses greater than 0.02 mg/kg.
Aminophylline to Reverse Effects
Methylxanthines, such as caffeine, aminophylline, and theophylline, are competitive adenosine receptor antagonists and aminophylline has been used to terminate persistent pharmacodynamic effects. Aminophylline may be administered in doses ranging from 50 mg to 250 mg by slow intravenous injection (50 mg to 100 mg over 30 to 60 seconds). Methylxanthine use is not recommended in patients who experience a seizure in association with regadenoson administration [see Warnings and Precautions (5.8)].
-
11 DESCRIPTION
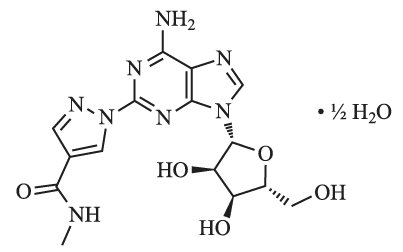
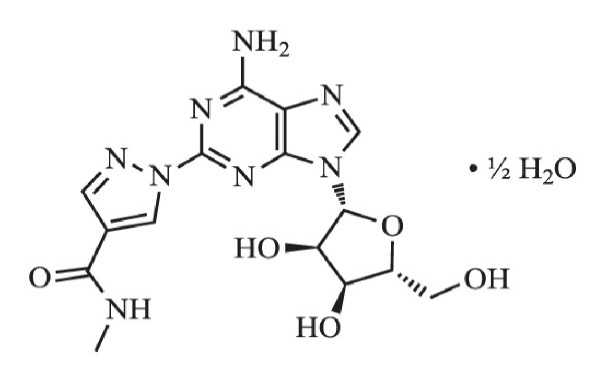
Regadenoson is an A2A adenosine receptor agonist that is a coronary vasodilator [see Clinical Pharmacology (12.1)]. Regadenoson is chemically described as adenosine, 2-[4-[(methylamino)carbonyl]-1H-pyrazol-1-yl]-, hemihydrate. Its structural formula is:
The molecular formula for regadenoson hemihydrate is C15H18N8O5 • ½H2O and its molecular weight is 399.35.
Regadenoson is a sterile, nonpyrogenic solution for intravenous injection. The solution is clear and colorless. Each 1 mL in the 5 mL pre-filled syringe contains 0.082 mg of regadenoson hemihydrate, corresponding to 0.08 mg regadenoson on an anhydrous basis, 10.9 mg dibasic sodium phosphate dihydrate, 5.4 mg monobasic sodium phosphate monohydrate, 150 mg propylene glycol, 1 mg edetate disodium dihydrate, and Water for Injection, with pH between 6.3 and 7.7.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Regadenoson is a low affinity agonist (Ki ≈ 1.3 mcM) for the A2A adenosine receptor, with at least 10-fold lower affinity for the A1 adenosine receptor (Ki > 16.5 mcM), and weak, if any, affinity for the A2B and A3 adenosine receptors. Activation of the A2A adenosine receptor by regadenoson produces coronary vasodilation and increases coronary blood flow (CBF).
12.2 Pharmacodynamics
Coronary Blood Flow
Regadenoson causes a rapid increase in CBF which is sustained for a short duration. In patients undergoing coronary catheterization, pulsed-wave Doppler ultrasonography was used to measure the average peak velocity (APV) of coronary blood flow before and up to 30 minutes after administration of regadenoson (0.4 mg, intravenously). Mean APV increased to greater than twice baseline by 30 seconds and decreased to less than twice the baseline level within 10 minutes [see Clinical Pharmacology (12.3)].
Myocardial uptake of the radiopharmaceutical is proportional to CBF. Because regadenoson increases blood flow in normal coronary arteries with little or no increase in stenotic arteries, regadenoson causes relatively less uptake of the radiopharmaceutical in vascular territories supplied by stenotic arteries. MPI intensity after regadenoson administration is therefore greater in areas perfused by normal relative to stenosed arteries.
Effect of duration of injection
A study in dogs compared the effects of intravenous injection of 2.5 mcg/kg regadenoson (in 10 mL) over 10 seconds and 30 seconds on CBF. The duration of a two-fold increase in CBF was 97±14 seconds (n=6) and 221±20 seconds (n=4), respectively, for the 10 second and 30 second injections. The peak effects (i.e., maximal increase) on CBF after the 10 second and 30 second injections were 217±15% and 297±33% above baseline, respectively. The times to peak effect on CBF were 17±2 seconds and 27±6 seconds, respectively.
Effect of Aminophylline
Aminophylline (100 mg, administered by slow intravenous injection over 60 seconds) injected 1 minute after 0.4 mg regadenoson in patients undergoing cardiac catheterization, was shown to shorten the duration of the coronary blood flow response to regadenoson as measured by pulsed-wave Doppler ultrasonography [see Overdosage (10)].
Effect of Caffeine
Ingestion of caffeine decreases the ability to detect reversible ischemic defects. In a placebo-controlled, parallel group clinical study, patients with known or suspected myocardial ischemia received a baseline rest/stress MPI followed by a second stress MPI. Patients received caffeine or placebo 90 minutes before the second regadenoson stress MPI. Following caffeine administration (200 or 400 mg), the mean number of reversible defects identified was reduced by approximately 60%. This decrease was statistically significant [see Drug Interactions (7.1) and Patient Counseling Information (17)].
Hemodynamic Effects
In clinical studies, the majority of patients had an increase in heart rate and a decrease in blood pressure within 45 minutes after administration of regadenoson. Maximum hemodynamic changes after regadenoson and adenosine in Studies 1 and 2 are summarized in Table 5.
Table 5 Hemodynamic Effects in Studies 1 and 2 Vital Sign Parameter
Regadenoson
N = 1,337
Adenosine
N = 678
Heart Rate
> 100 bpm
22%
13%
Increase > 40 bpm
5%
3%
Systolic Blood Pressure
< 90 mm Hg
2%
3%
Decrease > 35 mm Hg
7%
8%
≥ 200 mm Hg
1.9%
1.9%
Increase ≥ 50 mm Hg
0.7%
0.8%
≥ 180 mm Hg and increase of ≥ 20 mm Hg from baseline
4.6%
3.2%
Diastolic Blood Pressure
< 50 mm Hg
2%
4%
Decrease > 25 mm Hg
4%
5%
≥ 115 mm Hg
0.9%
0.9%
Increase ≥ 30 mm Hg
0.5%
1.1%
Hemodynamic Effects Following Inadequate Exercise
In a clinical study, regadenoson was administered for MPI following inadequate exercise stress. More patients with regadenoson administration three minutes following inadequate exercise stress had an increase in heart rate and a decrease in systolic blood pressure compared with regadenoson administered at rest. The changes were not associated with any clinically significant adverse reactions. Maximum hemodynamic changes are presented in Table 6.
Table 6 Hemodynamic Effects in Inadequate Exercise Stress Study
Vital Sign Parameter
Group 1 / MPI 1
Regadenoson
3 minutes following exercise
(N=575)
Group 2 / MPI 1
Regadenoson
1 hour following exercise
(N=567)
Heart Rate
> 100 bpm
44%
31%
Increase > 40 bpm
5%
16%
Systolic Blood Pressure
< 90 mm Hg
2%
4%
Decrease > 35 mm Hg
29%
10%
≥ 200 mm Hg
0.9%
0.4%
Increase ≥ 50 mm Hg
2%
0.4%
≥ 180 mm Hg and increase of ≥ 20 mm Hg from baseline
5%
2%
Diastolic Blood Pressure
< 50 mm Hg
3%
3%
Decrease > 25 mm Hg
6%
5%
≥ 115 mm Hg
0.7%
0.4%
Increase ≥ 30 mm Hg
2%
1%
Respiratory Effects
The A2B and A3 adenosine receptors have been implicated in the pathophysiology of bronchoconstriction in susceptible individuals (i.e., asthmatics). In in vitro studies, regadenoson has not been shown to have appreciable binding affinity for the A2B and A3 adenosine receptors.
In a randomized, placebo-controlled clinical trial of 999 patients with a diagnosis, or risk factors for, coronary artery disease and concurrent asthma or COPD, the incidence of respiratory adverse reactions (dyspnea, wheezing) was greater with regadenoson compared to placebo. Moderate (2.5%) or severe (<1%) respiratory reactions were observed more frequently in the regadenoson group compared to placebo [see Adverse Reactions (6.1)].
12.3 Pharmacokinetics
In healthy subjects, the regadenoson plasma concentration-time profile is multi-exponential in nature and best characterized by 3-compartment model. The maximal plasma concentration of regadenoson is achieved within 1 to 4 minutes after injection of regadenoson and parallels the onset of the pharmacodynamic response. The half-life of this initial phase is approximately 2 to 4 minutes. An intermediate phase follows, with a half-life on average of 30 minutes coinciding with loss of the pharmacodynamic effect. The terminal phase consists of a decline in plasma concentration with a half-life of approximately 2 hours [see Clinical Pharmacology (12.2)]. Within the dose range of 0.3 to 20 mcg/kg in healthy subjects, clearance, terminal half-life or volume of distribution do not appear dependent upon the dose.
A population pharmacokinetic analysis including data from subjects and patients demonstrated that regadenoson clearance decreases in parallel with a reduction in creatinine clearance and clearance increases with increased body weight. Age, gender, and race have minimal effects on the pharmacokinetics of regadenoson.
Specific Populations
Renally Impaired Patients: The disposition of regadenoson was studied in 18 patients with various degrees of renal function and in 6 healthy subjects. With increasing renal impairment, from mild (CLcr 50 to < 80 mL/min) to moderate (CLcr 30 to < 50 mL/min) to severe renal impairment (CLcr < 30 mL/min), the fraction of regadenoson excreted unchanged in urine and the renal clearance decreased, resulting in increased elimination half-lives and AUC values compared to healthy subjects (CLcr ≥ 80 mL/min). However, the maximum observed plasma concentrations as well as volumes of distribution estimates were similar across the groups. The plasma concentration-time profiles were not significantly altered in the early stages after dosing when most pharmacologic effects are observed. No dose adjustment is needed in patients with renal impairment.
Patients with End Stage Renal Disease: The pharmacokinetics of regadenoson in patients on dialysis has not been assessed; however, in an in vitro study regadenoson was found to be dialyzable.
Hepatically Impaired Patients: The influence of hepatic impairment on the pharmacokinetics of regadenoson has not been evaluated. Because greater than 55% of the dose is excreted in the urine as unchanged drug and factors that decrease clearance do not affect the plasma concentration in the early stages after dosing when clinically meaningful pharmacologic effects are observed, no dose adjustment is needed in patients with hepatic impairment.
Geriatric Patients: Based on a population pharmacokinetic analysis, age has a minor influence on the pharmacokinetics of regadenoson. No dose adjustment is needed in elderly patients.
Metabolism
The metabolism of regadenoson is unknown in humans. Incubation with rat, dog, and human liver microsomes as well as human hepatocytes produced no detectable metabolites of regadenoson.
Excretion
In healthy volunteers, 57% of the regadenoson dose is excreted unchanged in the urine (range 19 to 77%), with an average plasma renal clearance around 450 mL/min, i.e., in excess of the glomerular filtration rate. This indicates that renal tubular secretion plays a role in regadenoson elimination.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Regadenoson was negative in the Ames bacterial mutation assay, chromosomal aberration assay in Chinese hamster ovary (CHO) cells, and mouse bone marrow micronucleus assay.
Long-term animal studies have not been conducted to evaluate regadenoson’s carcinogenic potential or potential effects on fertility.
13.2 Animal Toxicology and/or Pharmacology
Cardiomyopathy
Minimal cardiomyopathy (myocyte necrosis and inflammation) was observed in rats following single-dose administration of regadenoson. Increased incidence of minimal cardiomyopathy was observed on day 2 in males at doses of 0.08, 0.2 and 0.8 mg/kg (1/5, 2/5, and 5/5) and in females (2/5) at 0.8 mg/kg. In a separate study in male rats, the mean arterial pressure was decreased by 30 to 50% of baseline values for up to 90 minutes at regadenoson doses of 0.2 and 0.8 mg/kg, respectively. No cardiomyopathy was noted in rats sacrificed 15 days following single administration of regadenoson. The mechanism of the cardiomyopathy induced by regadenoson was not elucidated in this study but was associated with the hypotensive effects of regadenoson. Profound hypotension induced by vasoactive drugs is known to cause cardiomyopathy in rats.
Local Irritation
Intravenous administration of regadenoson to rabbits resulted in perivascular hemorrhage, vein vasculitis, inflammation, thrombosis and necrosis, with inflammation and thrombosis persisting through day 8 (last observation day). Perivascular administration of regadenoson to rabbits resulted in hemorrhage, inflammation, pustule formation and epidermal hyperplasia, which persisted through day 8 except for the hemorrhage which resolved. Subcutaneous administration of regadenoson to rabbits resulted in hemorrhage, acute inflammation, and necrosis; on day 8 muscle fiber regeneration was observed.
-
14 CLINICAL STUDIES
Agreement between Regadenoson and Adenosine
The efficacy and safety of regadenoson were determined relative to adenosine in two randomized, double-blind studies (Studies 1 and 2) in 2,015 patients with known or suspected coronary artery disease who were indicated for pharmacologic stress MPI. A total of 1,871 of these patients had images considered valid for the primary efficacy evaluation, including 1,294 (69%) men and 577 (31%) women with a median age of 66 years (range 26 to 93 years of age). Each patient received an initial stress scan using adenosine (6-minute infusion using a dose of 0.14 mg/kg/min, without exercise) with a radionuclide gated SPECT imaging protocol. After the initial scan, patients were randomized to either regadenoson or adenosine, and received a second stress scan with the same radionuclide imaging protocol as that used for the initial scan. The median time between scans was 7 days (range of 1 to 104 days).
The most common cardiovascular histories included hypertension (81%), CABG, PTCA or stenting (51%), angina (63%), and history of myocardial infarction (41%) or arrhythmia (33%); other medical history included diabetes (32%) and COPD (5%). Patients with a recent history of serious uncontrolled ventricular arrhythmia, myocardial infarction, or unstable angina, a history of greater than first-degree AV block, or with symptomatic bradycardia, sick sinus syndrome, or a heart transplant were excluded. A number of patients took cardioactive medications on the day of the scan, including ß-blockers (18%), calcium channel blockers (9%), and nitrates (6%). In the pooled study population, 68% of patients had 0 to 1 segments showing reversible defects on the initial scan, 24% had 2 to 4 segments, and 9% had ≥ 5 segments.
Comparison of the images obtained with regadenoson to those obtained with adenosine was performed as follows. Using the 17-segment model, the number of segments showing a reversible perfusion defect was calculated for the initial adenosine study and for the randomized study obtained using regadenoson or adenosine. The agreement rate for the image obtained with regadenoson or adenosine relative to the initial adenosine image was calculated by determining how frequently the patients assigned to each initial adenosine category (0 to 1, 2 to 4, 5 to 17 reversible segments) were placed in the same category with the randomized scan. The agreement rates for regadenoson and adenosine were calculated as the average of the agreement rates across the three categories determined by the initial scan. Studies 1 and 2 each demonstrated that regadenoson is similar to adenosine in assessing the extent of reversible perfusion abnormalities (Table 7).
Table 7 Agreement Rates in Studies 1 and 2 Study 1
Study 2
Adenosine – Adenosine Agreement Rate (± SE)
61 ± 3%
64 ± 4%
Adenosine – Regadenoson Agreement Rate (± SE)
62 ± 2%
63 ± 3%
Rate Difference (Regadenoson – Adenosine) (± SE)
95% Confidence Interval
1 ± 4%
-7.5, 9.2%
-1 ± 5%
-11.2, 8.7%
Use of Regadenoson in Patients with Inadequate Exercise Stress
The efficacy and safety of regadenoson administered 3 minutes (Group 1) or 1 hour (Group 2) following inadequate exercise stress were evaluated in an open-label randomized, multi-center, non-inferiority study. Adequate exercise was defined as ≥ 85% maximum predicted heart rate and ≥ 5 METS. SPECT MPI was performed 60 to 90 minutes after regadenoson administration in each group (MPI 1). Patients returned 1 to 14 days later to undergo a second stress MPI with regadenoson without exercise (MPI 2).
All patients were referred for evaluation of coronary artery disease. Of the 1,147 patients randomized, a total of 1,073 patients received regadenoson and had interpretable SPECT scans at all visits; 538 in Group 1 and 535 in Group 2. The median age of the patients was 62 years (range 28 to 90 years) and included 633 (59%) men and 440 (41%) women.
Images from MPI 1 and MPI 2 for the two groups were compared for presence or absence of perfusion defects. The level of agreement between the MPI 1 and the MPI 2 reads in Group 1 was similar to the level of agreement between MPI 1 and MPI 2 reads in Group 2. However, two patients receiving regadenoson 3 minutes following inadequate exercise experienced a serious cardiac adverse reaction. No serious cardiac adverse reactions occurred in patients receiving regadenoson 1 hour following inadequate exercise stress [see Adverse Reactions (6.1), Clinical Pharmacology (12.2)].
-
16 HOW SUPPLIED/STORAGE AND HANDLING
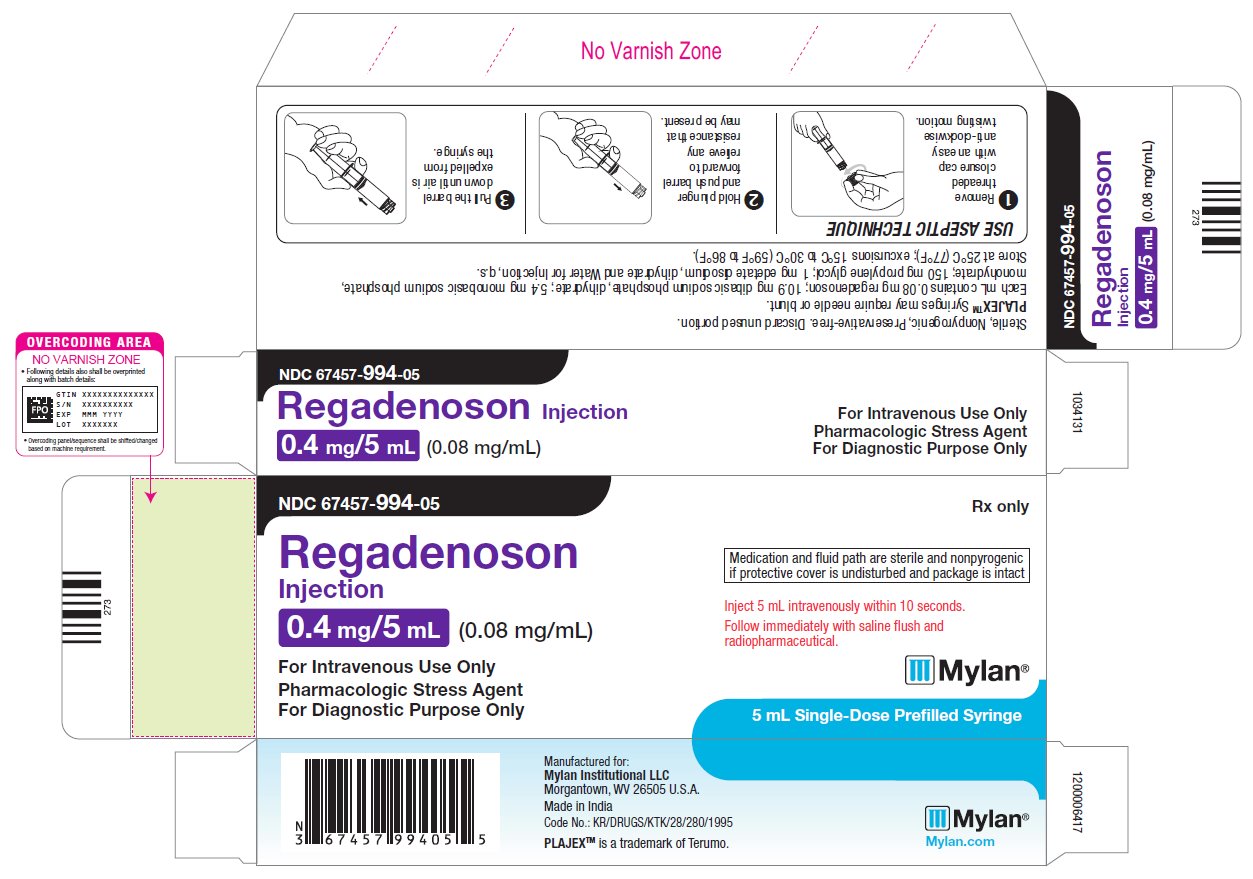
Regadenoson injection is supplied as a sterile, preservative-free solution containing 0.08 mg/mL regadenoson in the following package:
- •
- Single-dose 5 mL pre-filled plastic PLAJEX™ syringes with luer-lock fitting (NDC 67457-994-05).
Store at controlled room temperature, 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
-
17 PATIENT COUNSELING INFORMATION
Drug Interaction
Patients should be instructed to avoid consumption of any products containing methylxanthines, including caffeinated coffee, tea or other caffeinated beverages, caffeine-containing drug products, aminophylline and theophylline for at least 12 hours before a scheduled radionuclide MPI [see Warnings and Precautions (5.8) and Clinical Pharmacology (12.2)].Cardiovascular
Advise patients that they may be at increased risk of fatal and nonfatal heart attacks, abnormal heart rhythms, cardiac arrest, significant increase or decrease in blood pressure, or cerebrovascular accidents (stroke) with the use of regadenoson [see Warnings and Precautions (5.1), (5.3), (5.5), (5.6) and (5.9)].Hypersensitivity
Inform patients that allergic reactions have been reported with regadenoson. Advise patients how to recognize such a reaction and when to seek medical attention [see Warnings and Precautions (5.4)].Respiratory
Advise patients with COPD or asthma about the need for administration of pre- and post-study bronchodilator therapy and to call their clinician if they experience any shortness of breath or difficulty breathing following an MPI study with regadenoson [see Warnings and Precautions (5.7)].Seizures
Advise patients that they may be at increased risk of seizures. Question patients about a history of seizures [see Warnings and Precautions (5.8)].Lactation
Advise a woman to pump and discard breast milk for 10 hours after regadenoson administration [see Use in Specific Populations (8.2)].PLAJEXTM is a trademark of Terumo. All other trademarks and registered trademarks are the property of their respective owners.
Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Manufactured by:
Steriscience Specialties Private Limited
Bangalore, India1033950
1200006419NOVEMBER 2022
-
PRINCIPAL DISPLAY PANEL – 0.4 mg/5 mL
NDC 67457-994-05
Regadenoson
Injection0.4 mg/5 mL (0.08 mg/mL)
For Intravenous Use Only
Pharmacologic Stress Agent
For Diagnostic Purpose OnlyRx only
Medication and fluid path are sterile and nonpyrogenic
if protective cover is undisturbed and package is intact
Inject 5 mL intravenously within 10 seconds.
Follow immediately with saline flush and
radiopharmaceutical.5 mL Single-Dose Prefilled Syringe
Sterile, Nonpyrogenic, Preservative-free. Discard unused portion.
PLAJEX™ Syringes may require needle or blunt.
Each mL contains 0.08 mg regadenoson; 10.9 mg dibasic sodium phosphate, dihydrate; 5.4 mg monobasic sodium phosphate,
monohydrate; 150 mg propylene glycol; 1 mg edetate disodium, dihydrate and Water for Injection, q.s.
Store at 25°C (77°F); excursions 15°C to 30°C (59°F to 86°F).Manufactured for:
Mylan Institutional LLC
Morgantown, WV 26505 U.S.A.Made in India
Code No.: KR/DRUGS/KTK/28/280/1995
PLAJEX™ is a trademark of Terumo.
Mylan.com
-
INGREDIENTS AND APPEARANCE
REGADENOSON
regadenoson injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67457-994 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength REGADENOSON (UNII: 2XLN4Y044H) (REGADENOSON ANHYDROUS - UNII:7AXV542LZ4) REGADENOSON ANHYDROUS 0.4 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: 94255I6E2T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67457-994-05 1 in 1 CARTON 09/21/2023 06/30/2026 1 5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA213856 09/21/2023 06/30/2026 Labeler - Mylan Institutional LLC (790384502)