Label: LEUKOTRAP - CP2D- anticoagulant citrate phosphate double dextrose solution
- NDC Code(s): 53157-455-02
- Packager: Haemonetics Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 13, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- HOW SUPPLIED
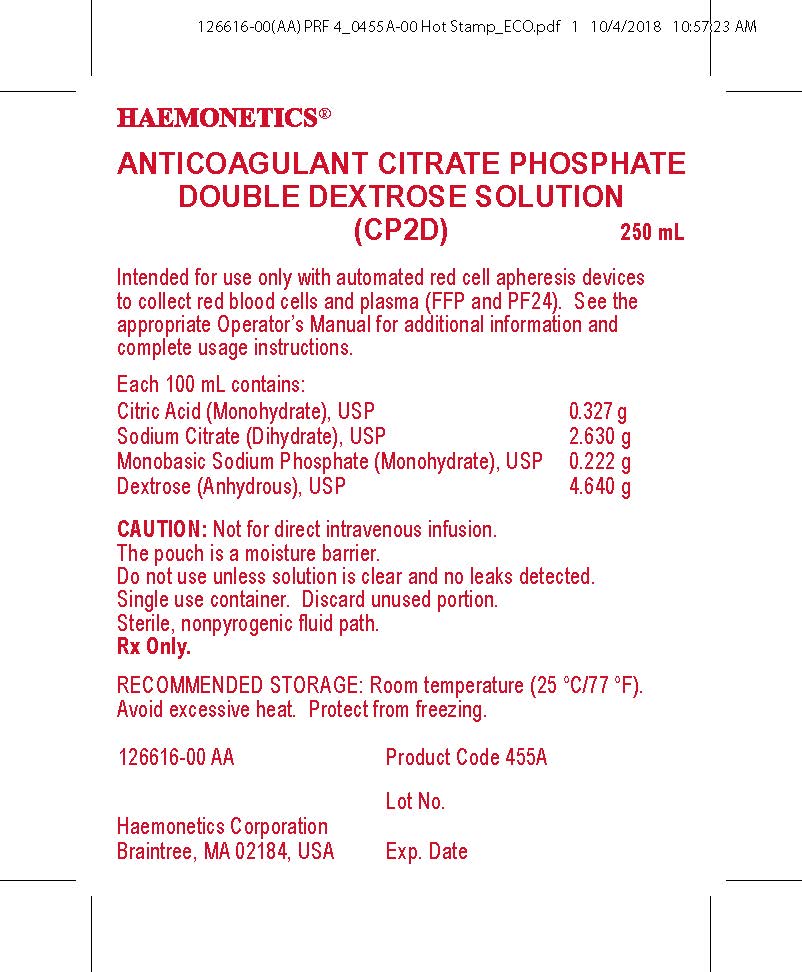
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEUKOTRAP - CP2D
anticoagulant citrate phosphate double dextrose solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:53157-455 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE, UNSPECIFIED FORM (UNII: IY9XDZ35W2) (DEXTROSE, UNSPECIFIED FORM - UNII:IY9XDZ35W2) DEXTROSE, UNSPECIFIED FORM 4.64 g in 100 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) 2.63 g in 100 mL CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 0.327 g in 100 mL SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) 0.222 g in 100 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:53157-455-02 250 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN820915 10/09/2019 Labeler - Haemonetics Corporation (057827420) Establishment Name Address ID/FEI Business Operations Haemonetics Manufacturing Inc 078598396 manufacture(53157-455)