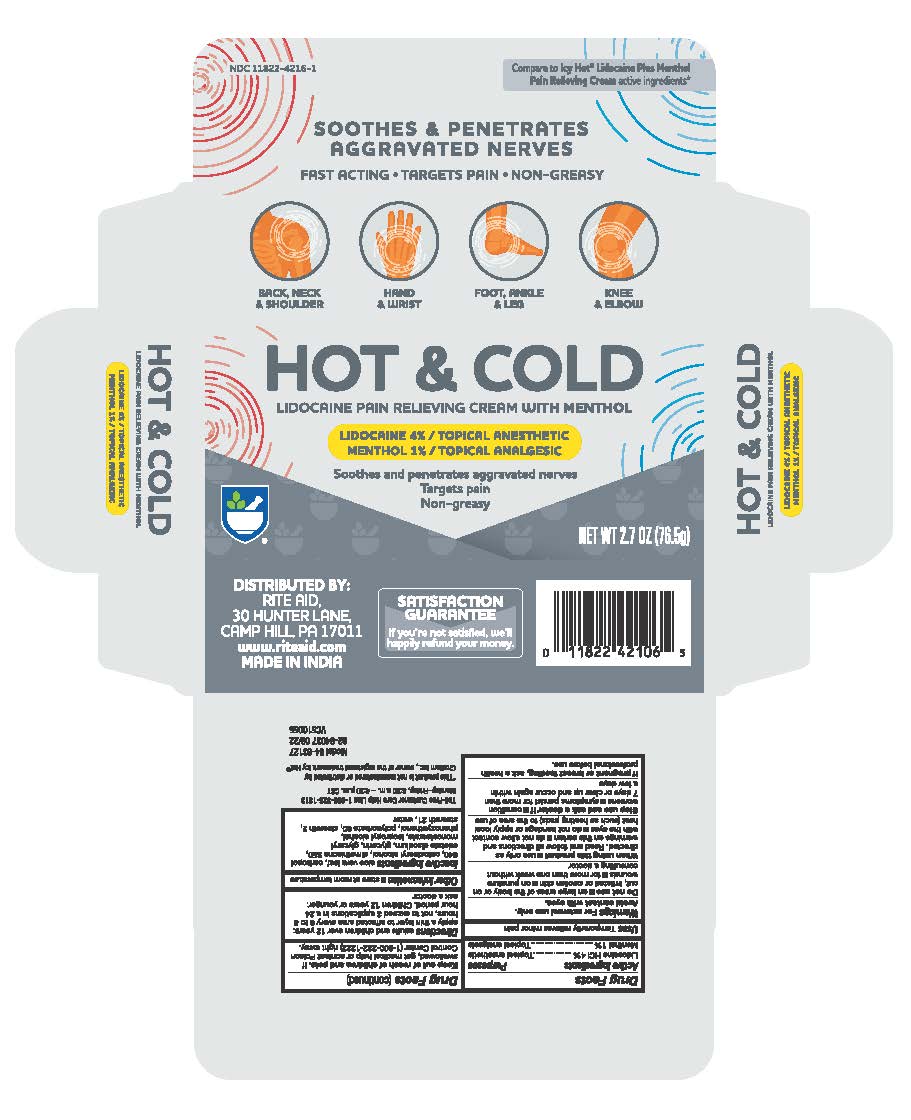
Label: RITE AID HOT AND COLD PAIN RELIEF PAIN RELIEVING- lidocaine hcl and menthol cream
- NDC Code(s): 11822-4216-1
- Packager: Rite Aid
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purposes
- Uses
-
Warnings
For external use only
Avoid contact with eyes
Do not use
- on large areas of the body or on cut, irritated or swollen skin
- on puncture wounds
- for more than one week without consulting a doctor
When using this product
- Use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contact with eyes
- do not bandage or apply local heat (such as heating pads) to the area of use
- on large areas of the body or on cut, irritated or swollen skin
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RITE AID HOT AND COLD PAIN RELIEF PAIN RELIEVING
lidocaine hcl and menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-4216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 0.4 g in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CARBOMER 940 (UNII: 4Q93RCW27E) DIMETHICONE 350 (UNII: 2Y53S6ATLU) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL ALCOHOL (UNII: ND2M416302) PHENOXYETHANOL (UNII: HIE492ZZ3T) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARETH-2 (UNII: V56DFE46J5) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYSORBATE 60 (UNII: CAL22UVI4M) STEARETH-21 (UNII: 53J3F32P58) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-4216-1 1 in 1 CARTON 06/01/2022 1 76.5 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/01/2022 Labeler - Rite Aid (014578892) Establishment Name Address ID/FEI Business Operations ANICARE PHARMACEUTICALS PRIVATE LIMITED 916837425 manufacture(11822-4216)