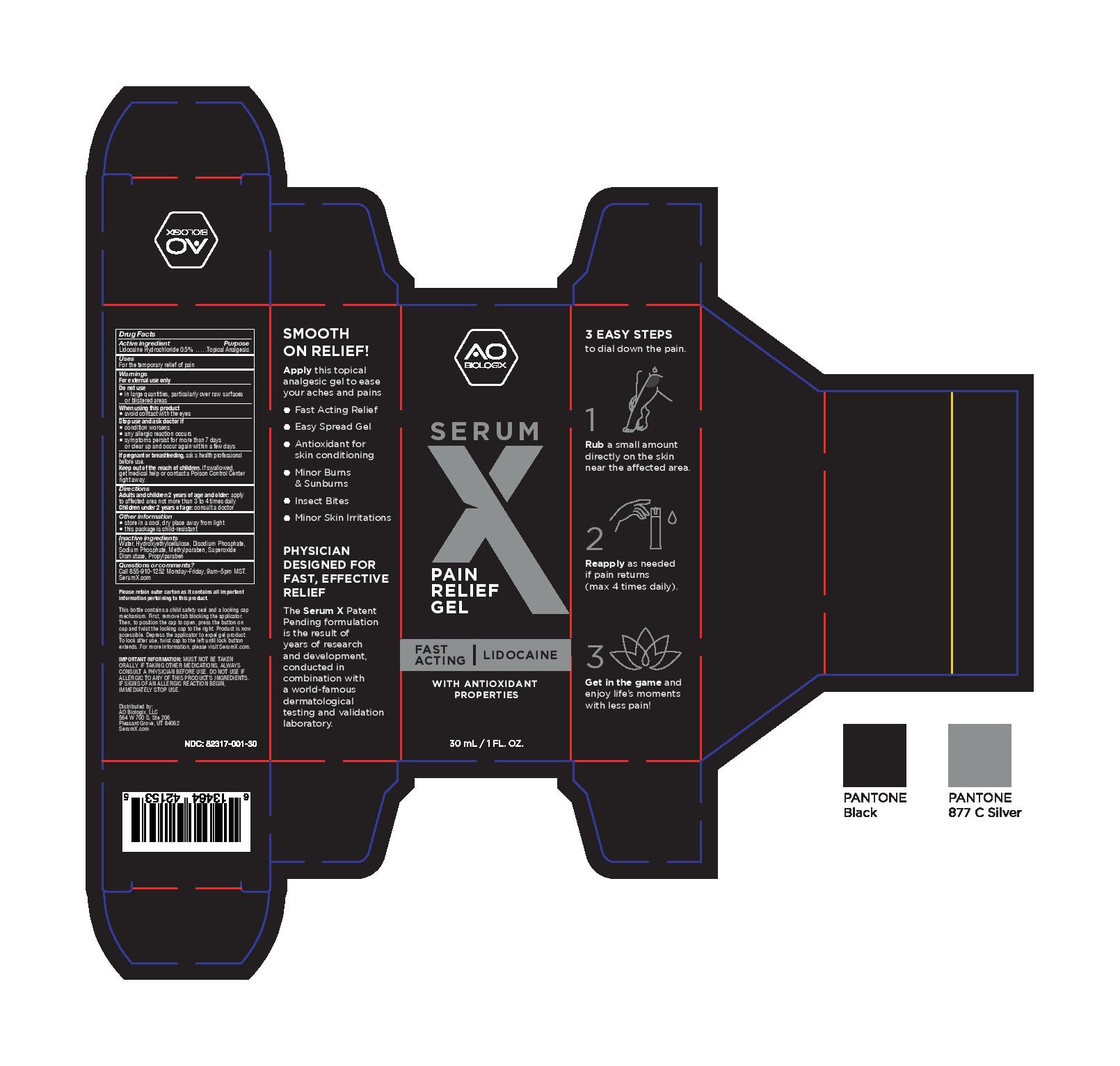
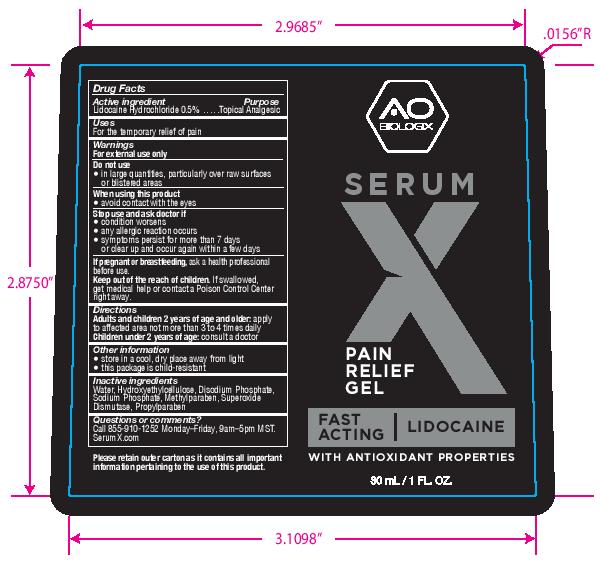
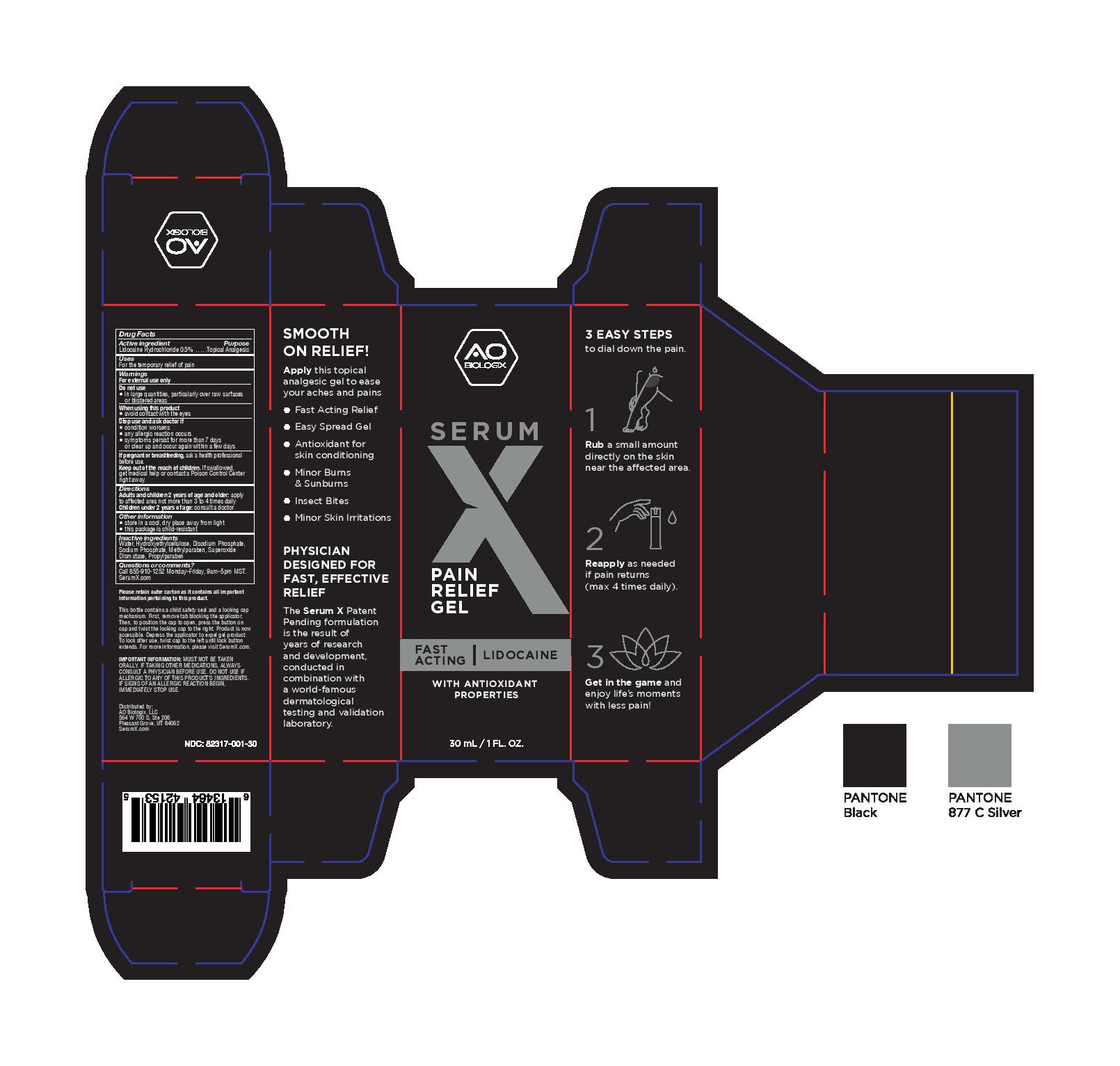
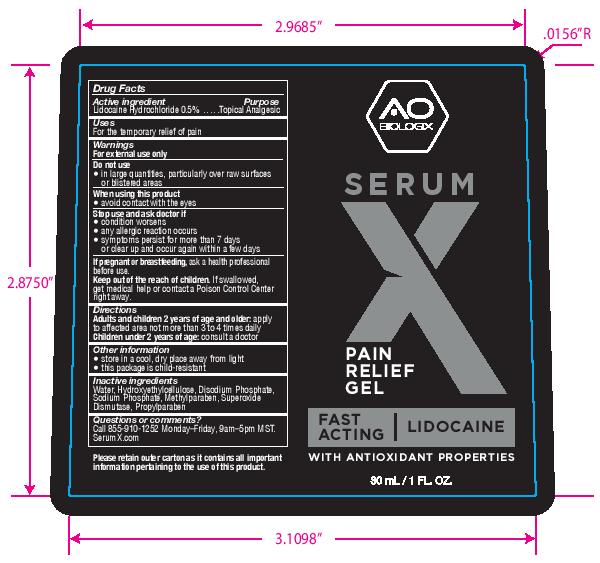
Label: SERUM X- lidocaine hydrochloride gel
- NDC Code(s): 82317-001-01, 82317-001-30
- Packager: AO Biologix, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SERUM X
lidocaine hydrochloride gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82317-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) SODIUM PHOSPHATE (UNII: SE337SVY37) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) METHYLPARABEN (UNII: A2I8C7HI9T) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SUPEROXIDE DISMUTASE (SACCHAROMYCES CEREVISIAE) (UNII: W2T4YRA9AD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82317-001-30 1 in 1 CARTON 04/21/2022 1 30 g in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:82317-001-01 1 in 1 PACKAGE 07/01/2022 2 0.5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 04/21/2022 Labeler - AO Biologix, LLC (102188692) Establishment Name Address ID/FEI Business Operations TRI-PAC, INC. 020844956 manufacture(82317-001)