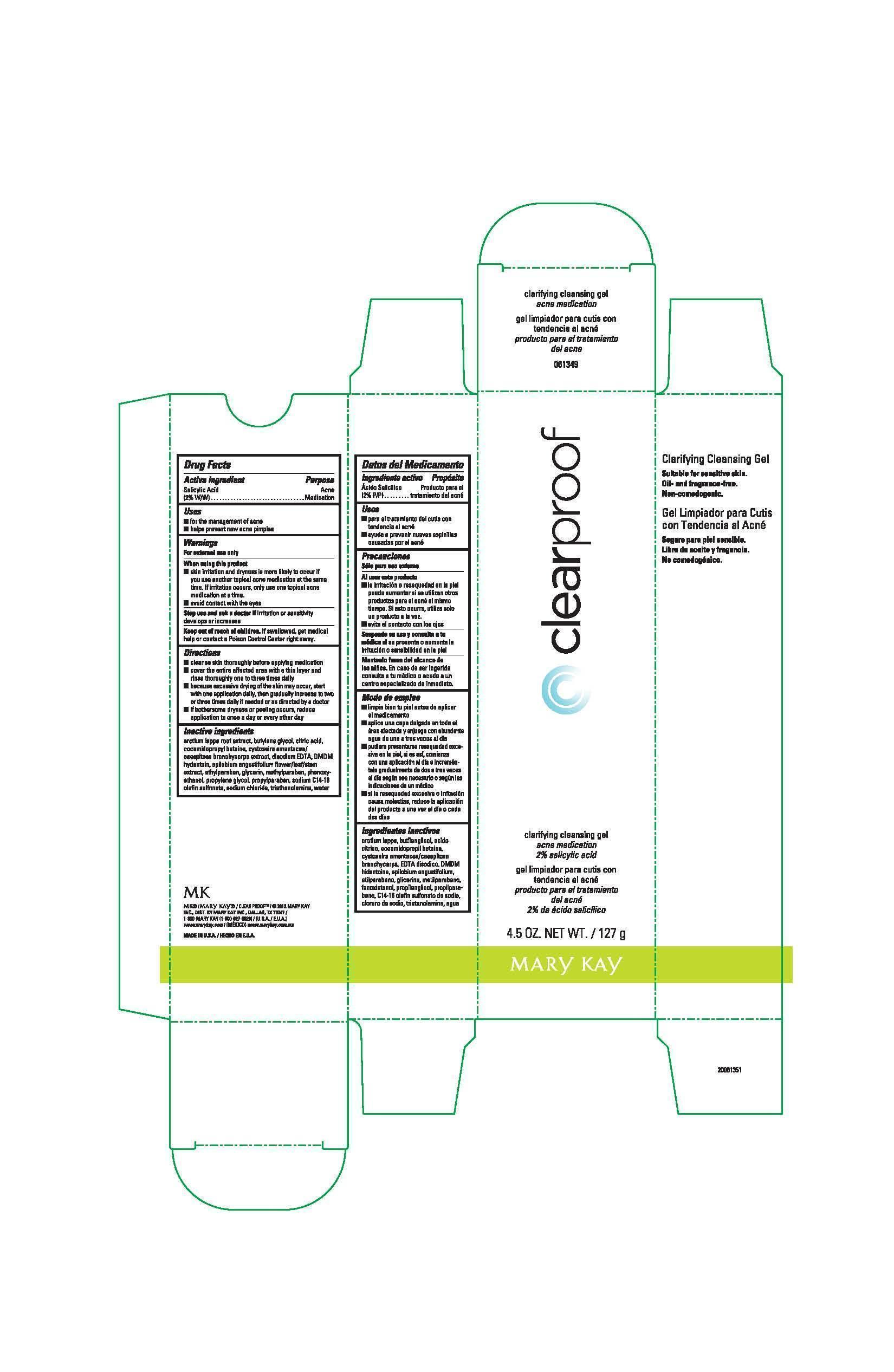
Label: CLEAR PROOF CLARIFYING CLEANSING GEL ACNE MEDICATION- salicylic acid gel
- NDC Code(s): 51531-1349-1, 51531-1349-4
- Packager: Mary Kay Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Uses
- Warnings
-
Directions
- cleanse skin thoroughly before applying medication
- cover the entire affected area with a thin layer and rinse thoroughly one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
-
Inactive ingredients
arctium lappa root extract, butylene glycol, citric acid, cocamidopropyl betaine, cystoseira amentacea/caespitosa branchycarpa extract, disodium EDTA, DMDM hydantoin, epilobium angustifolium flower/leaf/stem extract, ethylparaben, glycerin, methylparaben, phenoxyethanol, propylene glycol, propylparaben, sodium C14-16 olefin sulfonate, sodium chloride, triethanolamine, water
- Principal Display Panel - 127 g carton
-
INGREDIENTS AND APPEARANCE
CLEAR PROOF CLARIFYING CLEANSING GEL ACNE MEDICATION
salicylic acid gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51531-1349 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) DMDM HYDANTOIN (UNII: BYR0546TOW) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) EPILOBIUM ANGUSTIFOLIUM FLOWERING TOP (UNII: 08H094218D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51531-1349-4 1 in 1 CARTON 08/15/2013 1 127 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:51531-1349-1 28 g in 1 TUBE; Type 0: Not a Combination Product 08/15/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/15/2013 Labeler - Mary Kay Inc. (049994452) Establishment Name Address ID/FEI Business Operations Port Jervis Laboratories Inc. 001535103 manufacture(51531-1349) Establishment Name Address ID/FEI Business Operations Mary Kay Inc. 103978839 manufacture(51531-1349) Establishment Name Address ID/FEI Business Operations Englewood Lab Inc. 172198223 pack(51531-1349)