Label: TRYVIO- aprocitentan tablet, film coated
- NDC Code(s): 80491-8012-3, 80491-8012-7, 80491-8012-8
- Packager: Idorsia Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TRYVIO safely and effectively. See full prescribing information for TRYVIO. TRYVIO™ (aprocitentan) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY
- TRYVIO is contraindicated for use during pregnancy because it may cause fetal harm if used by pregnant patients.Therefore in patients who can become pregnant, exclude pregnancy prior to initiation of TRYVIO.
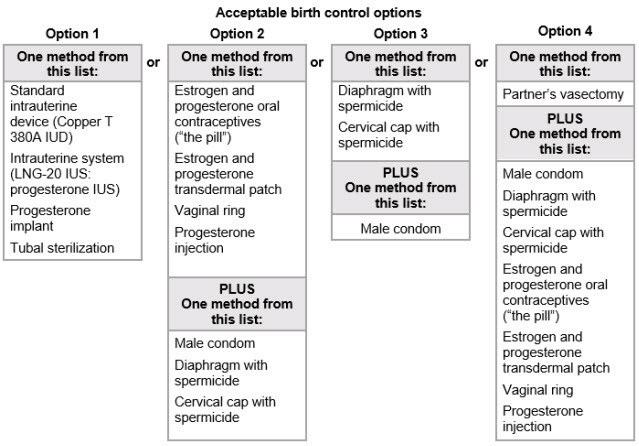
- Advise use of effective contraception before the start of TRYVIO, during treatment and for one month after stopping treatment.
- When pregnancy is detected, discontinue TRYVIO as soon as possible [see Dosage and Administration (2.2), Contraindications (4.1), Warnings and Precautions (5.1), Use in Specific Populations (8.1)].
-
1 INDICATIONS AND USAGETRYVIO, in combination with other antihypertensive drugs, is indicated for the treatment of hypertension, to lower blood pressure (BP) in adult patients who are not adequately controlled on other ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of TRYVIO is 12.5 mg orally once daily. Swallow tablets whole. TRYVIO may be taken with or without food. If a dose is missed, skip the missed dose ...
-
3 DOSAGE FORMS AND STRENGTHSTRYVIO (aprocitentan) tablets are available as: 12.5 mg: yellow to orange round, film-coated tablet, debossed with "AN" on one side and plain on the other side.
-
4 CONTRAINDICATIONS4.1 Pregnancy - Use of TRYVIO is contraindicated in patients who are pregnant [see Dosage and Administration (2.2), Warnings and Precautions (5.1) and Use in Specific Populations ...
-
5 WARNINGS AND PRECAUTIONS5.1 Embryo-Fetal Toxicity - Based on data from animal reproduction studies with endothelin receptor antagonists (ERAs), TRYVIO may cause fetal harm when administered during pregnancy and is ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the labeling include: Embryo-fetal toxicity [see Warnings and Precautions (5.1)] Hepatotoxicity [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on animal reproduction studies with other ERAs, TRYVIO can cause embryo-fetal toxicity, including birth defects and fetal death when administered to a ...
-
10 OVERDOSAGETRYVIO has been administered as a single dose of up to 600 mg, and as multiple doses of up to 100 mg daily, to healthy subjects (48 and 8 times the recommended dose, respectively). Adverse events ...
-
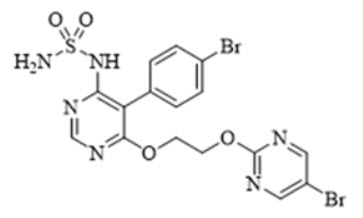
11 DESCRIPTIONTRYVIO (aprocitentan) is an endothelin receptor antagonist. The chemical name of aprocitentan is N-[5-(4-bromophenyl)-6- [2-[(5-bromo-2-pyrimidinyl)oxy]ethoxy]-4-pyrimidinyl]-sulfamide. It has a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Aprocitentan is an ERA that inhibits the binding of endothelin (ET)-1 to ETA and ETB receptors. ET-1, via its receptors (ETA and ETB), mediates a variety of deleterious ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Two-year carcinogenicity studies with macitentan (for which aprocitentan is a major metabolite) did not identify ...
-
14 CLINICAL STUDIESThe efficacy of TRYVIO (aprocitentan) was evaluated in a multipart, phase 3 multicenter study (PRECISION, NCT03541174) in adults with SBP ≥140 mmHg who were prescribed at least three ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - TRYVIO tablets are available as: 12.5 mg: yellow to orange round, film-coated tablet, debossed with "AN" on one side and plain on the other side. – NDC 80491-8012-8, each ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Embryo-Fetal Toxicity - Counsel patients who can become pregnant to use effective methods of contraception ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. IDRSTRMG03272025Issued: X/2025 - MEDICATION GUIDE - TRYVIO™ (try-vee-oh) (aprocitentan) tablets, for oral ...
-
PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle CartonNDC 80491-8012-3 - TRYVIO™ (aprocitentan) tablets - 12.5 mg - Attention: Dispense the enclosed - Medication Guide to each patient. Rx only - 30 film-coated tablets - idorsia
-
INGREDIENTS AND APPEARANCEProduct Information