Label: TAZAROTENE cream
- NDC Code(s): 45802-706-94, 45802-706-96
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 31, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAZAROTENE CREAM safely and effectively. See full prescribing information for TAZAROTENE CREAM. TAZAROTENE cream, 0.05% for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Plaque Psoriasis - Tazarotene cream, 0.05% is indicated for the topical treatment of patients with plaque psoriasis.
-
2 DOSAGE AND ADMINISTRATION 2.1 Important Administration Instructions - Tazarotene cream is for topical use only. Tazarotene cream is not for ophthalmic, oral, or intravaginal use. If contact with mucous membranes occurs ...
-
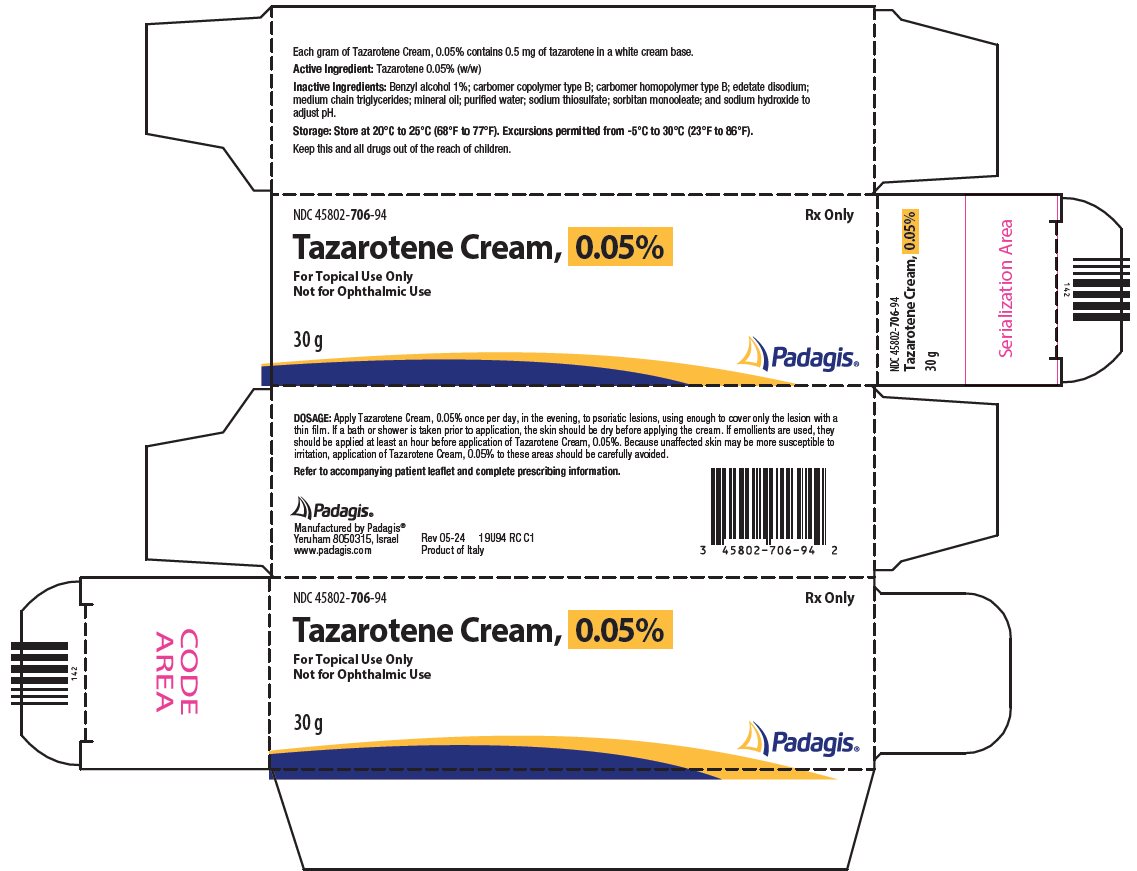
3 DOSAGE FORMS AND STRENGTHS Cream, 0.05%. Each gram of Tazarotene Cream, 0.05% contains 0.5 mg of tazarotene in a white cream base.
-
4 CONTRAINDICATIONS Tazarotene cream is contraindicated in: • Pregnancy. Retinoids may cause fetal harm when administered to a pregnant female [see Warnings and Precautions (5.1), Use in Specific Populations (8.1 ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Embryofetal Toxicity - Systemic exposure to tazarotenic acid is dependent upon the extent of the body surface area treated. In patients treated topically over sufficient body surface area ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed in more detail in other sections of the labeling: • Embryofetal toxicity [see Warnings and Precautions (5.1)] • Photosensitivity and Risk of ...
-
7 DRUG INTERACTIONS No formal drug-drug interaction studies were conducted with tazarotene cream. In a trial of 27 healthy female subjects between the ages of 20–55 years receiving a combination oral contraceptive ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on data from animal reproduction studies, retinoid pharmacology, and the potential for systemic absorption, tazarotene cream may cause fetal harm when ...
-
10 OVERDOSAGE Excessive topical use of tazarotene cream, 0.05% may lead to marked redness, peeling, or discomfort [see Warnings and Precautions (5.2)]. Tazarotene cream, 0.05% is not for oral use. Oral ...
-
11 DESCRIPTION Tazarotene Cream, 0.05% is for topical use and contains the active ingredient, tazarotene. Each gram of Tazarotene Cream, 0.05% contains 0.5 mg of tazarotene in a white cream base. Tazarotene is a ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Tazarotene is a retinoid prodrug which is converted to its active form, the carboxylic acid of tazarotene, by deesterification. Tazarotenic acid binds to all three ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - A long-term study of tazarotene following oral administration of 0.025, 0.050, and 0.125 mg/kg/day to rats showed no ...
-
14 CLINICAL STUDIES In two 12-week vehicle-controlled clinical trials, tazarotene cream, 0.05% and 0.1% was significantly more effective than vehicle in reducing the severity of stable plaque psoriasis. Tazarotene ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Tazarotene cream is a white cream available in a concentration of 0.05%. It is supplied in a collapsible aluminum tube with a tamper-evident aluminum membrane over the opening and a white ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information). Embryofetal Toxicity - Inform females of reproductive potential of the potential risk to a fetus. Advise these ...
-
Patient Information PATIENT INFORMATION - Tazarotene (taz ar’ oh teen) Cream, 0.05% Important information: tazarotene cream is for use on skin only. Do not use tazarotene cream in your eyes, mouth, or ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 45802-706-94 - Rx Only - Tazarotene Cream, 0.05% For Topical Use Only - Not for Ophthalmic Use - 30g
-
INGREDIENTS AND APPEARANCEProduct Information