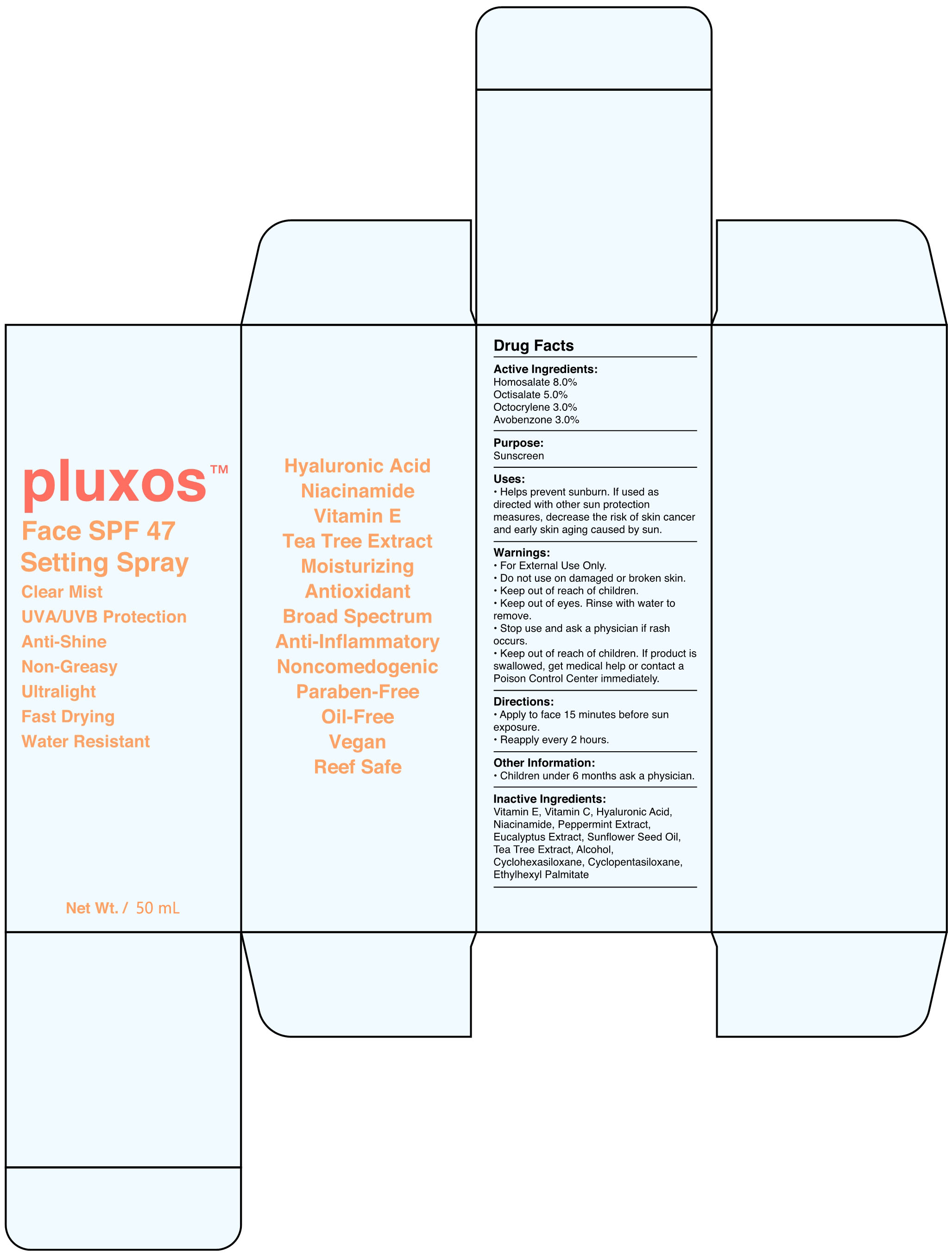
Label: FACE SPF 47 SETTING- face spf 47 setting spray spray
- NDC Code(s): 82723-001-01
- Packager: Aopline Health Industry Technology (Guangzhou) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
-
Warnings
For External Use Only.
Do not use on damaged or broken skin.
Keep out of reach of children.
Keep out of eyes. Rinse with water to remove.
Stop use and ask a physician if rash occurs.
Keep out of reach of children . If product is swallowed, get medical help or contact a Poison Control Center immediately.
- Directions
- Other Information
- Inactive Ingredients
- Keep out of reach of children.
- Stop use
-
WHEN USING
For External Use Only.
Do not use on damaged or broken skin.
Keep out of reach of children.
Keep out of eyes. Rinse with water to remove.
Stop use and ask a physician if rash occurs.
Keep out of reach of children . If product is swallowed, get medical help or contact a Poison Control Center immediately.
- Do not use
- Package Label
-
INGREDIENTS AND APPEARANCE
FACE SPF 47 SETTING
face spf 47 setting spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82723-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 mL Inactive Ingredients Ingredient Name Strength EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) SUNFLOWER OIL (UNII: 3W1JG795YI) ALCOHOL (UNII: 3K9958V90M) CYCLOMETHICONE 6 (UNII: XHK3U310BA) MELALEUCA ALTERNIFOLIA LEAF (UNII: G43C57162K) ETHYLHEXYL PALMITATE (UNII: 2865993309) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) HYALURONIC ACID (UNII: S270N0TRQY) PEPPERMINT (UNII: V95R5KMY2B) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) NIACINAMIDE (UNII: 25X51I8RD4) ASCORBIC ACID (UNII: PQ6CK8PD0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82723-001-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/07/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/07/2022 Labeler - Aopline Health Industry Technology (Guangzhou) Co., Ltd. (715076108) Establishment Name Address ID/FEI Business Operations Aopline Health Industry Technology (Guangzhou) Co., Ltd. 715076108 manufacture(82723-001)