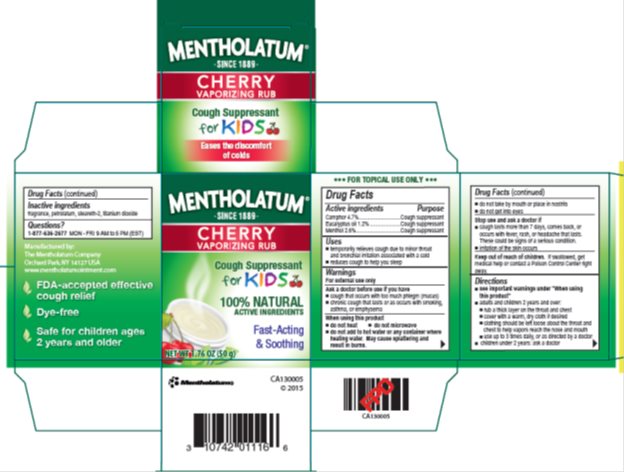
Label: MENTHOLATUM CHERRY VAPORIZING RUB- camphor, eucalyptus oil, menthol ointment
- NDC Code(s): 10742-8731-1
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts or as occurs with smoking, asthma, or emphysema
When using this product
- do not heat
- do not microwave
- do not add to hot water or any container where heating water. May cause splattering and result in burns.
- do not take by mouth or place in nostrils
- do not get into eyes
-
Directions
- see important warnings under "When using this product"
- adults and children 2 years and over:
- rub a thick layer on throat and chest
- cover with a warm, dry cloth if desired
- clothing should be left loose about the throat and chest to help vapors reach the nose and mouth
- use up to 3 times daily, or as directed by a doctor
- children under 2 years: ask a doctor
- Inactive ingredients
- Questions?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
MENTHOLATUM CHERRY VAPORIZING RUB
camphor, eucalyptus oil, menthol ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-8731 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (NATURAL) (UNII: N20HL7Q941) (CAMPHOR (NATURAL) - UNII:N20HL7Q941) CAMPHOR (NATURAL) 47 mg in 1 g EUCALYPTUS OIL (UNII: 2R04ONI662) (EUCALYPTUS OIL - UNII:2R04ONI662) EUCALYPTUS OIL 12 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 26 mg in 1 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) STEARETH-2 (UNII: V56DFE46J5) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-8731-1 1 in 1 CARTON 02/16/2015 1 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 02/16/2015 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-8731)