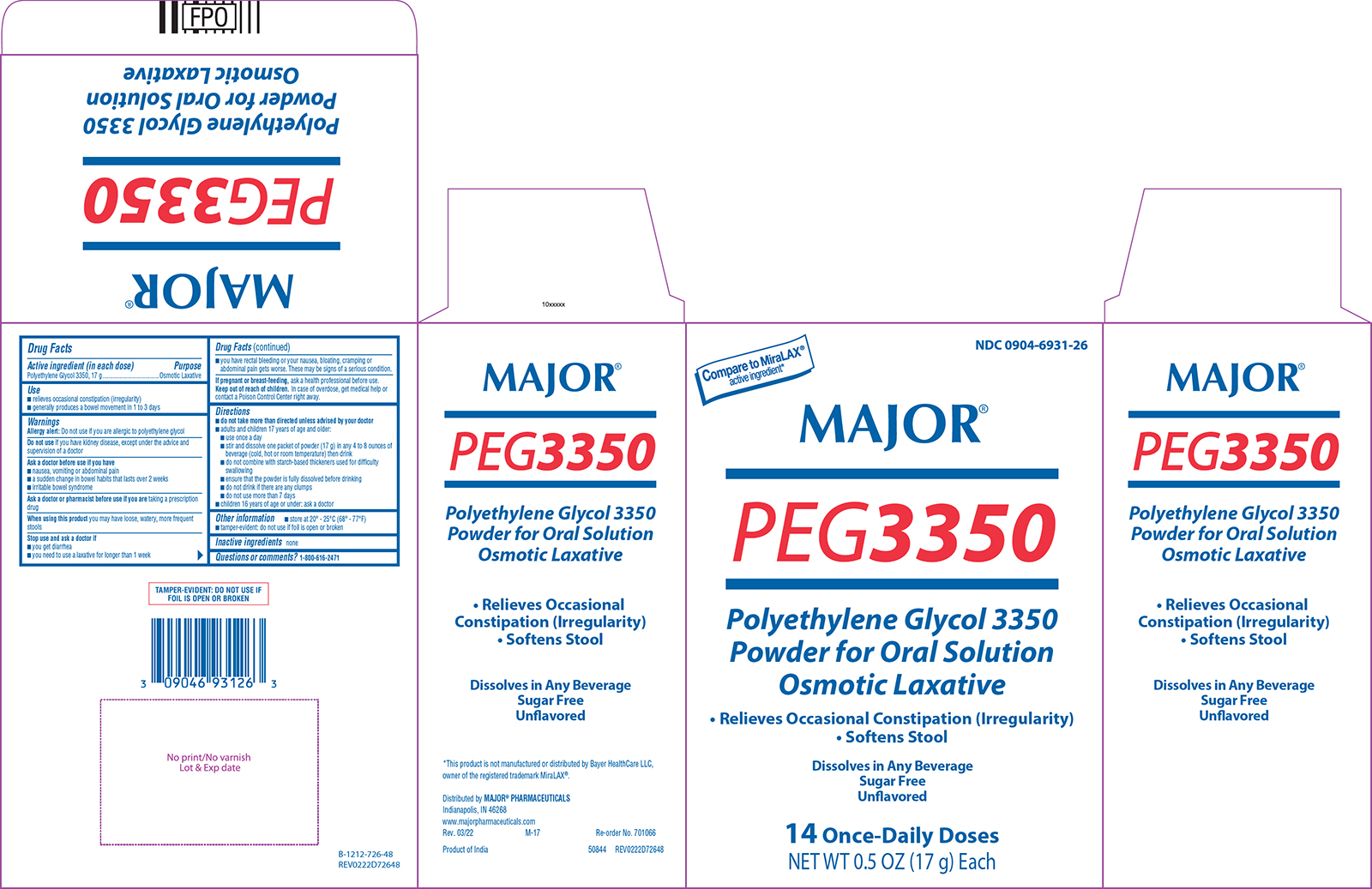
Label: POLYETHYLENE GLYCOL 3350 powder, for solution
- NDC Code(s): 0904-6931-26, 0904-6931-76, 0904-6931-81, 0904-6931-86
- Packager: Major Pharmaceuticals
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 29, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each dose)
Polyethylene Glycol 3350, 17 g
-
Purpose
Osmotic Laxative
-
Use
relieves occasional constipation (irregularity) generally produces a bowel movement in 1 to 3 days
-
Warnings
Allergy alert: Do not use if you are allergic to polyethylene glycol - Do not use - if you have kidney disease, except under the advice and supervision of a doctor - Ask a doctor before use ...
-
Directions
do not take more than directed unless advised by your doctor - adults and children 17 years of age and older: use once a day - stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of ...
-
Other information
store at 20° - 25°C (68° - 77°F) tamper-evident: do not use if foil is open or broken
-
Inactive ingredients
none
-
Questions or comments?
1-800-616-2471
-
Principal display panel
NDC 0904-6931-26 - Compare to MiraLAX® active ingredient* MAJOR® PEG3350 - Polyethylene Glycol 3350 - Powder for Oral Solution - Osmotic Laxative - • Relieves Occasional Constipation ...
-
INGREDIENTS AND APPEARANCEProduct Information