Label: MEGESTROL ACETATE tablet
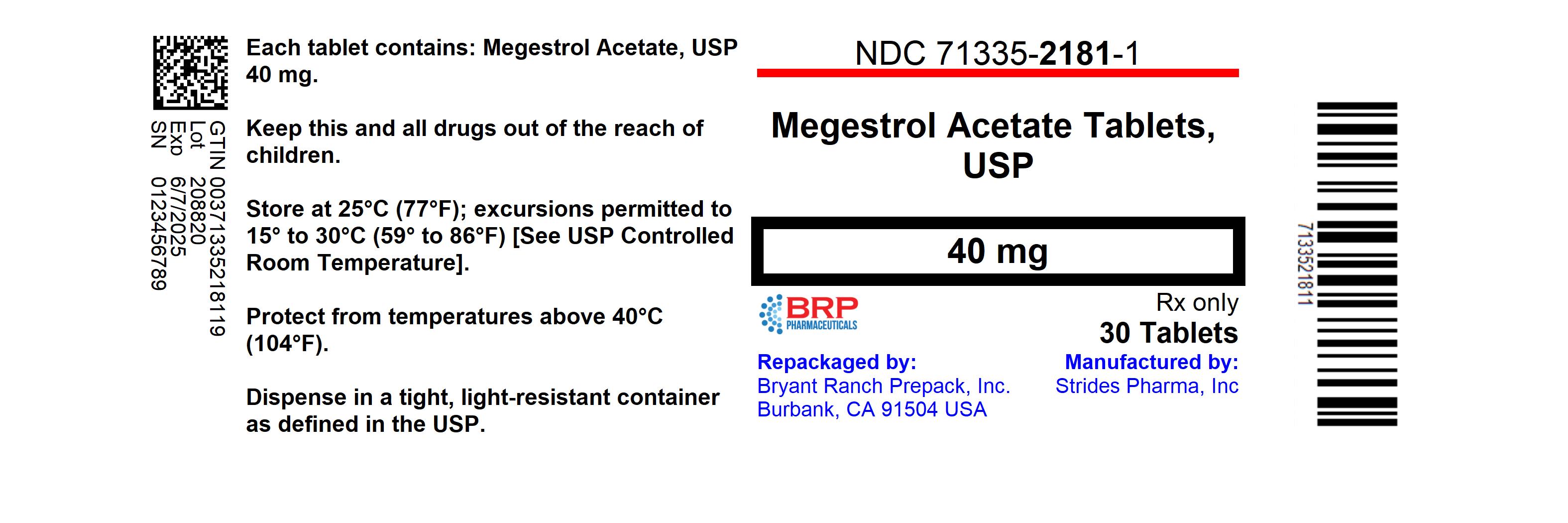
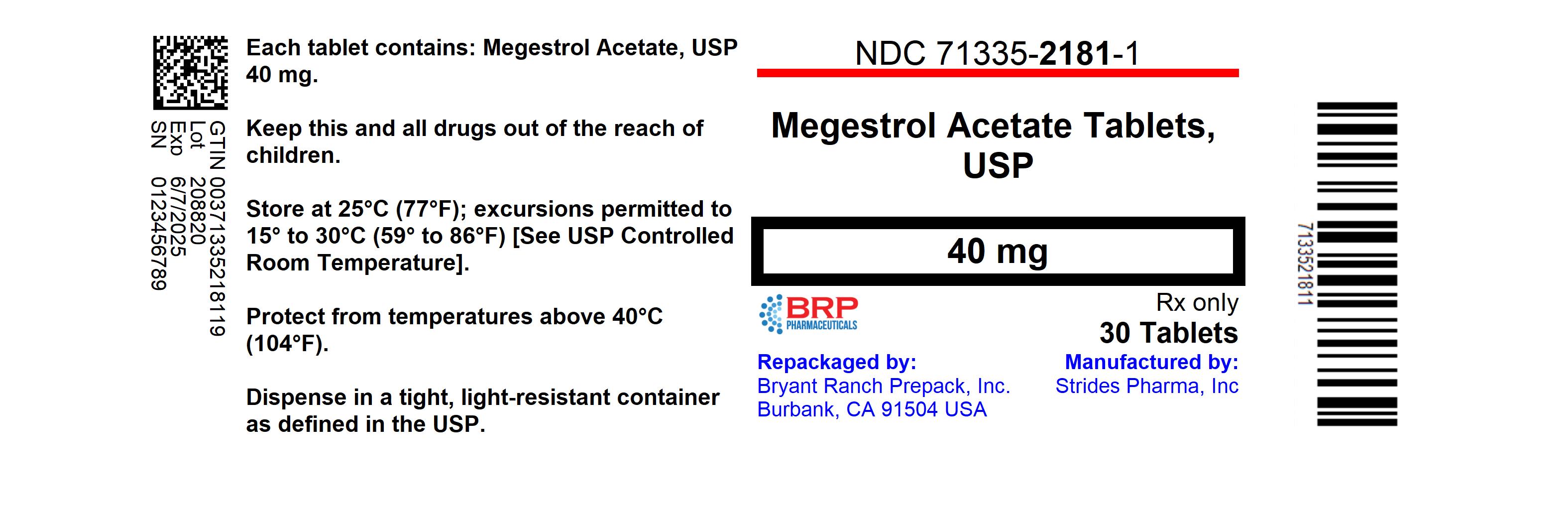
- NDC Code(s): 71335-2181-1, 71335-2181-2, 71335-2181-3
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 64380-159
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
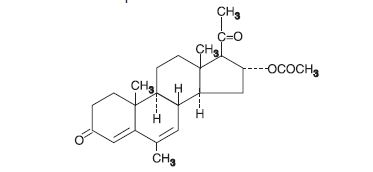
DESCRIPTIONMegestrol acetate is a synthetic, antineoplastic and progestational drug. Megestrol acetate is a white, crystalline solid chemically designated as ...
-
CLINICAL PHARMACOLOGYWhile the precise mechanism by which megestrol produces its antineoplastic effects against endometrial carcinoma is unknown at the present time, inhibition of pituitary gonadotropin production ...
-
INDICATIONS AND USAGEMegestrol acetate tablets are indicated for the palliative treatment of advanced carcinoma of the breast or endometrium (ie, recurrent, inoperable, or metastatic disease). It should not be used ...
-
CONTRAINDICATIONSHistory of hypersensitivity to megestrol acetate or any component of the formulation.
-
WARNINGSMegestrol acetate may cause fetal harm when administered to a pregnant woman. Fertility and reproduction studies with high doses of megestrol acetate have shown a reversible feminizing effect on ...
-
PRECAUTIONSGeneral - Close surveillance is indicated for any patient treated for recurrent or metastatic cancer. Use with caution in patients with a history of thromboembolic disease. Use in ...
-
ADVERSE REACTIONSWeight Gain - Weight gain is a frequent side effect of megestrol. This gain has been associated with increased appetite and is not necessarily associated with fluid retention. Thromboembolic ...
-
OVERDOSAGENo serious unexpected side effects have resulted from studies involving megestrol acetate administered in dosages as high as 1600 mg/day. Oral administration of large, single doses of megestrol ...
-
DOSAGE AND ADMINISTRATIONBreast cancer: 160 mg/day (40 mg q.i.d.). Endometrial carcinoma: 40 to 320 mg/day in divided doses. At least 2 months of continuous treatment is considered an adequate period ...
-
HOW SUPPLIEDMegestrol acetate tablets, 40 mg, are white, round, flat-faced, beveled-edged, bisected, debossed with "Par 290" on one side. NDC: 71335-2181-3: 15 TABLETs in a BOTTLE - NDC: 71335-2181-1: 30 ...
-
SPL UNCLASSIFIED SECTIONSPECIAL HANDLING - Health Hazard Data - There is no threshold limit value established by OSHA, NIOSH, or ACGIH. Exposure or "overdose" at levels approaching recommended dosing levels could result ...
-
PRINCIPAL DISPLAY PANELMegestrol Acetate 40mg Tablet
-
INGREDIENTS AND APPEARANCEProduct Information