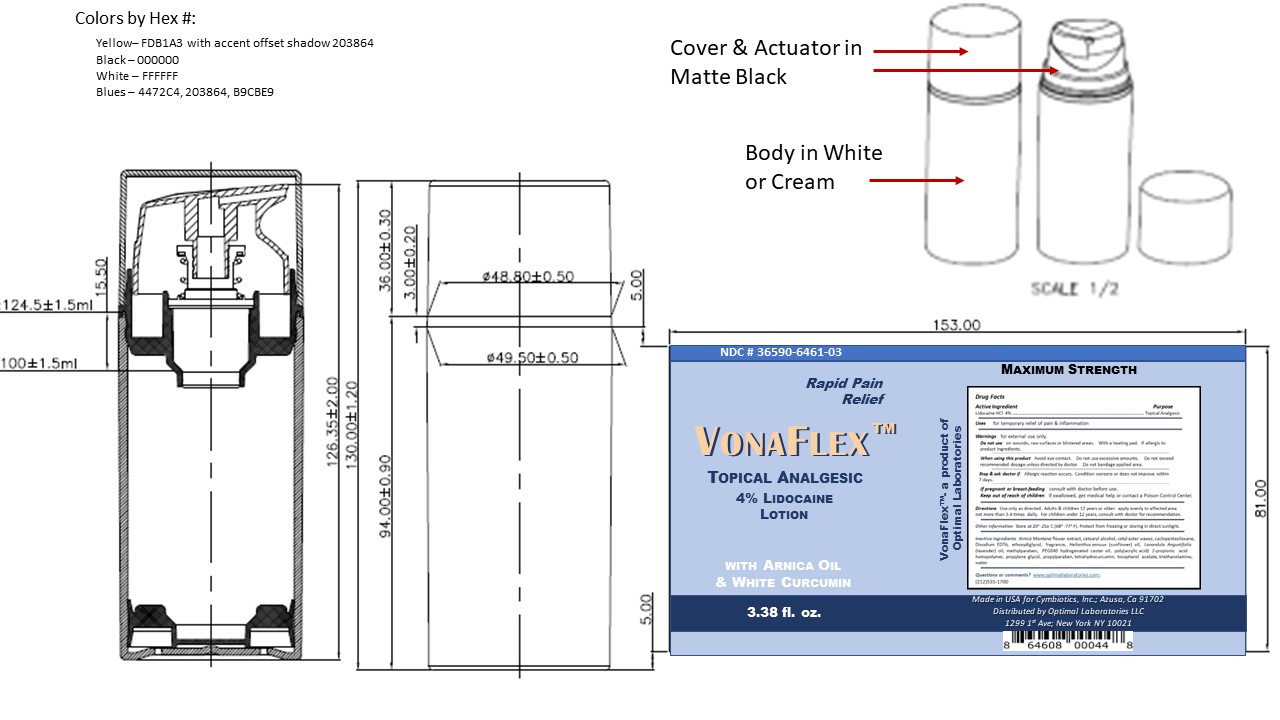
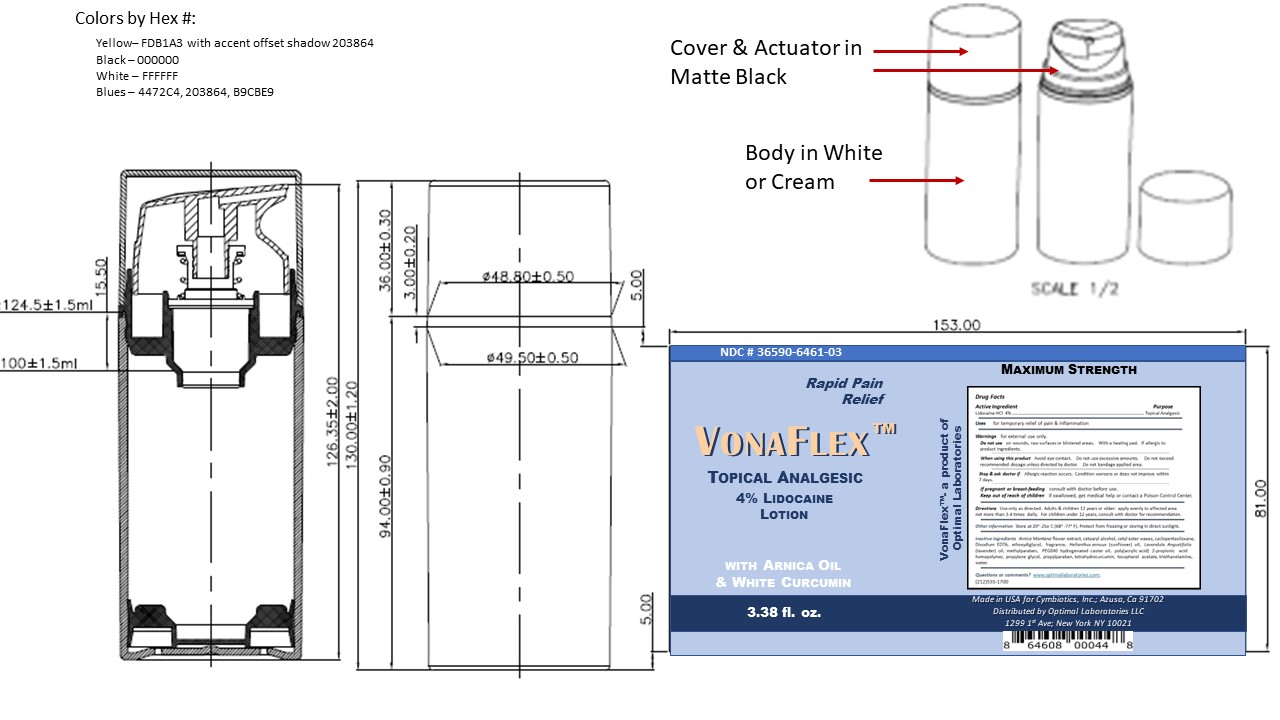
Label: VONAFLEX- lidocaine lotion lotion
-
NDC Code(s):
36590-6461-1,
36590-6461-2,
36590-6461-3,
36590-6461-4, view more36590-6461-5
- Packager: Cymbiotics, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Uses
- Directions
-
WARNINGS
For External use only.
Do not use:on wounds, raw surfaces or blistered areas, with a heating pad, or if allergic to product ingredients
When using this product:Avoid eye contact; do not use excessive amounts; do not exceed recommended dosage unless directed by doctor; do not bandage applied area.
Stop and ask doctor if:an allergic reaction occurs; condition worsens or does not improve within 7 days.
If pregnant or breast-feeding:consult with doctor before use.
if swallowed, get medical help or contact a Poison Control Center.
- Other Information:
-
INACTIVE INGREDIENT
Arnica Montanaflower extract, cetearyl alcohol, cetyl ester waxes, cyclopentasiloxane, disodium EDTA, ethoxydiglycol, fragrance, Helianthus annuus(sunflower) oil, Lavandula Angustifolio(lavender) oil, methylparaben, PEG40 hydrogenated caster oil, poly(acrylic acid) 2-propionic acid homopolymer, propylene glycol, propylparaben, tetrahydrocurcumin, tocopherol acetate, triethanolamine, water.
- Questions and Comments
- Package Label and Principal Display Panel Vonaflex
-
INGREDIENTS AND APPEARANCE
VONAFLEX
lidocaine lotion lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:36590-6461 Route of Administration TOPICAL, CUTANEOUS, VAGINAL, RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength METHYLPARABEN (UNII: A2I8C7HI9T) 0.2 g in 100 g EDETATE DISODIUM (UNII: 7FLD91C86K) 0.1 g in 100 g PROPYLPARABEN (UNII: Z8IX2SC1OH) 0.02 g in 100 g WATER (UNII: 059QF0KO0R) 71.93 g in 100 g SUNFLOWER OIL (UNII: 3W1JG795YI) 0.75 g in 100 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.55 g in 100 g LAVENDER OIL (UNII: ZBP1YXW0H8) 1 g in 100 g POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) 1 g in 100 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 4 g in 100 g DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) 5 g in 100 g CETYL ESTERS WAX (UNII: D072FFP9GU) 4 g in 100 g CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) 2 g in 100 g TETRAHYDRODIFERULOYLMETHANE (UNII: 00U0645U03) 2 g in 100 g TROLAMINE (UNII: 9O3K93S3TK) 1.5 g in 100 g .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) 1.2 g in 100 g CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) 0.4 g in 100 g FRAGRANCE LAVENDER ROSE ORC1004596 (UNII: 1XW43TV4PI) 0.1 g in 100 g ARNICA MONTANA FLOWER WATER (UNII: U7L2JP51PR) 0.25 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:36590-6461-1 50 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 2 NDC:36590-6461-2 75 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 3 NDC:36590-6461-3 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/26/2022 4 NDC:36590-6461-4 150 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 08/26/2022 5 NDC:36590-6461-5 200 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/25/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/25/2022 Labeler - Cymbiotics, Inc (781766709) Registrant - Westwood Laboratories, LLC (832280635) Establishment Name Address ID/FEI Business Operations Westwood Laboratories, LLC 832280635 manufacture(36590-6461) , label(36590-6461) , pack(36590-6461)