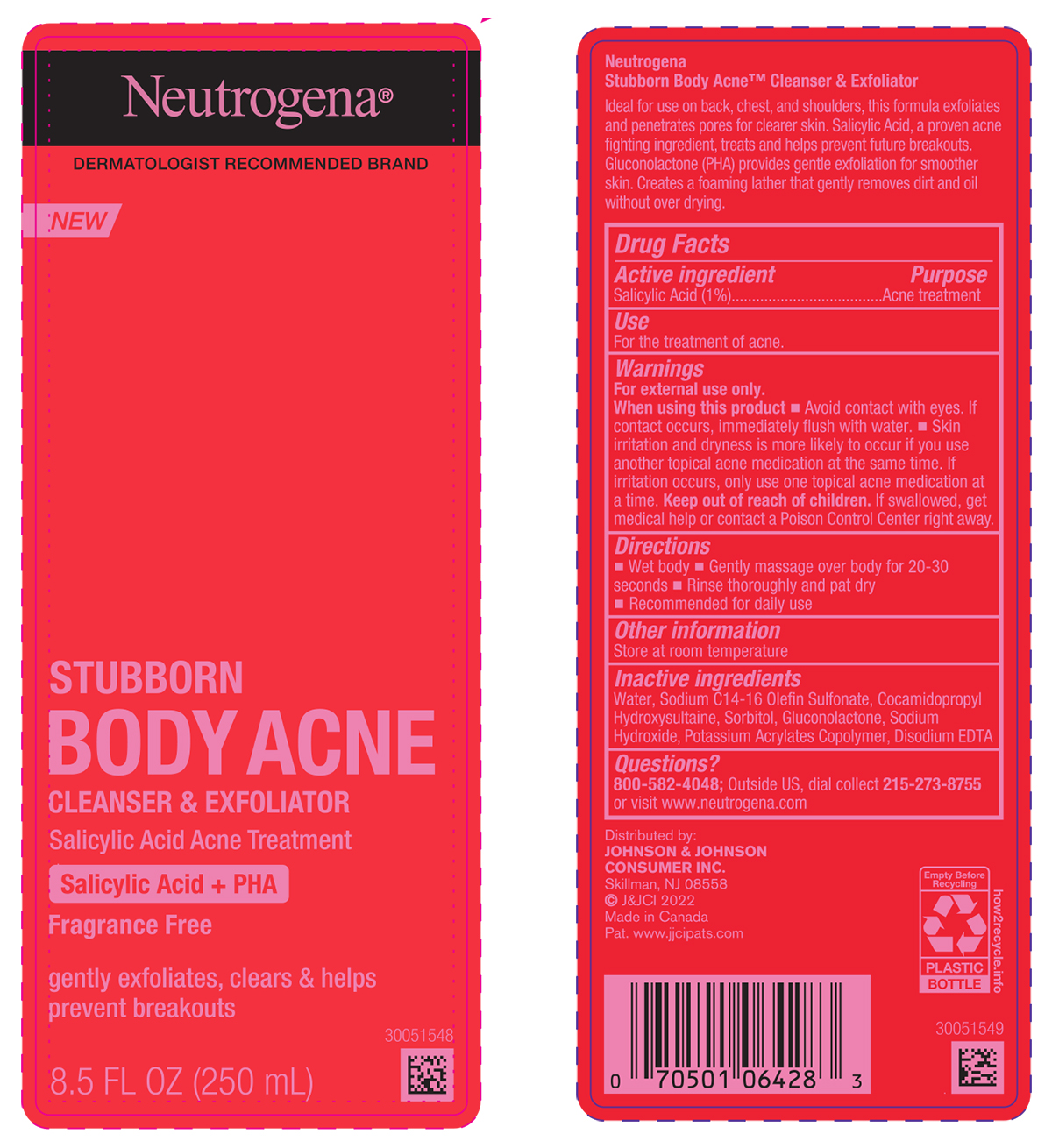
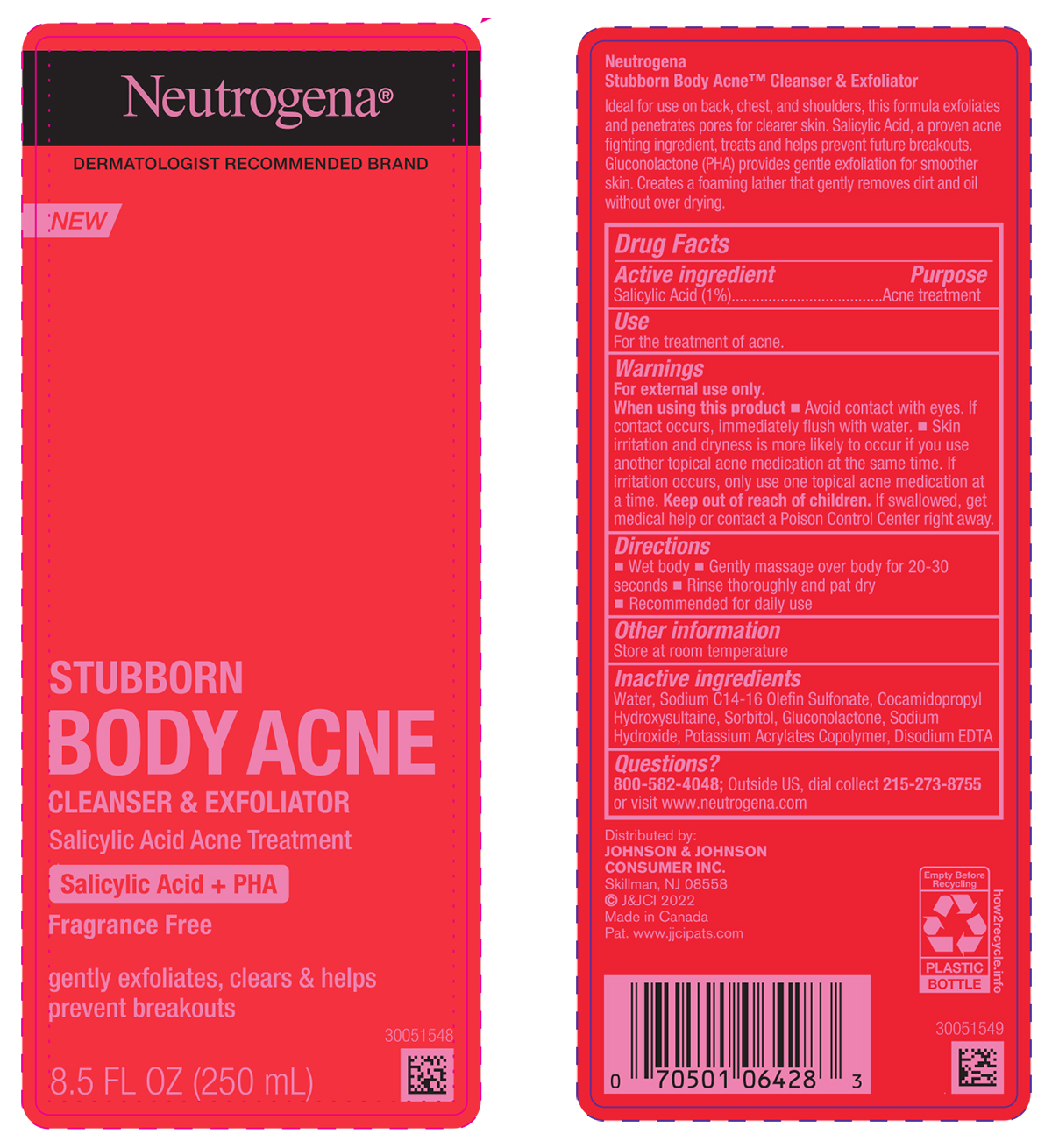
Label: NEUTROGENA STUBBORN BODY ACNE CLEANSER AND EXFOLIATOR- salicylic acid liquid
- NDC Code(s): 69968-0759-8
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
- Directions
- Other Information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 250mL Bottle
-
INGREDIENTS AND APPEARANCE
NEUTROGENA STUBBORN BODY ACNE CLEANSER AND EXFOLIATOR
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0759 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLUCONOLACTONE (UNII: WQ29KQ9POT) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0759-8 250 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/01/2022 Labeler - Kenvue Brands LLC (118772437)