Label: DIGOXIN solution
- NDC Code(s): 17856-0057-1, 17856-0057-5
- Packager: Atlantic Biologicals Corps
- This is a repackaged label.
- Source NDC Code(s): 0054-0057
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated May 27, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONDigoxin Oral Solution - These highlights do not include all the information needed to use Digoxin Oral Solution safely and effectively. See full prescribing information for Digoxin Oral Solution ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Heart Failure - Digoxin Oral Solution, USP is indicated for the treatment of mild to moderate heart failure. Digoxin increases left ventricular ejection fraction and improves heart failure ...
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Considerations - The dose of digoxin should be based on clinical assessment but individual patient factors should be taken into consideration. Those factors are: Lean body ...
-
3 DOSAGE FORMS AND STRENGTHSEach 1 mL of clear, colorless Digoxin Oral Solution contains 0.05 mg (50 mcg). The Digoxin Oral Solution bottles are to be used with the graduated droppers provided in the carton. Starting at 0.2 ...
-
4 CONTRAINDICATIONSAllergy to digoxin is rare. Digoxin is contraindicated in patients with a known hypersensitivity to digoxin or other forms of digitalis. Digitalis glycosides, such as digoxin, are contraindicated ...
-
5 WARNINGS AND PRECAUTIONS5.1 Use in Patients with Accessory AV Pathway (Wolff-Parkinson-White Syndrome) Patients with Wolff-Parkinson-White syndrome who develop atrial fibrillation are at high risk of ventricular ...
-
6 ADVERSE REACTIONSThe frequency and severity of adverse reactions to digoxin when taken orally depend on the dose and the patient's underlying disease or concomitant therapies The overall incidence of adverse ...
-
7 DRUG INTERACTIONS7.1 P-Glycoprotein (PGP) Inducers/Inhibitors - Digoxin is a substrate for P-glycoprotein, at the level of intestinal absorption, renal tubular section and biliary-intestinal secretion. Therefore ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Teratogenic Effects (Pregnancy Category C) Animal reproduction studies have not been conducted with digoxin. It is also not known whether digoxin can cause fetal harm when ...
-
10 OVERDOSAGE10.1 Clinical Manifestations - In adults, the signs and symptoms of toxicity are similar to those described in but may be more frequent and severe. The most common signs and symptoms of digoxin ...
-
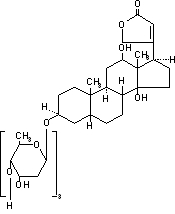
11 DESCRIPTIONDigoxin is one of the cardiac glycosides, a closely-related group of plant-derived drugs with shared pharmacological effects. The term "digitalis" is used to designate the whole group. Digoxin is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - All of digoxin’s actions are mediated through its effects on NaK–ATPase. This enzyme, the “sodium pump,” is responsible for maintaining the intracellular milieu ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - There have been no long-term studies performed in animals to evaluate carcinogenic potential, nor have studies been conducted to assess ...
-
14 CLINICAL STUDIES14.1 Chronic Heart Failure - Two small12-week, double-blind, randomized trials compared digoxin to placebo in adult patients with chronic congestive heart failure, New York Heart Association ...
-
17 PATIENT COUNSELING INFORMATIONPatients receiving digoxin should be given the following instructions by the physician. Advise patients that digoxin is a cardiac glycoside used to treat heart failure and heart arrhythmias ...
-
DIGOXIN SOLUTION

-
INGREDIENTS AND APPEARANCEProduct Information