Label: DYE FREE ADULT COUGH PLUS CHEST CONGESTION DM- dextromethorphan hydrobromide and guaifenesin solution
- NDC Code(s): 70000-0651-1
- Packager: CARDINAL HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 ml)
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- ▪
- cough that occurs with too much phlegm (mucus)
- ▪
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
-
DOSAGE & ADMINISTRATION
Directions
- ▪
- do not take more than 6 doses in any 24-hour period
- ▪
- measure only with dosing cup provided
- ▪
- keep dosing cup with product
- ▪
- ml = milliliter
- ▪
- this adult product is not intended for use in children under 12 years of age
age
dose
adults and children 12 years and over
20 ml every 4 hours
children under 12 years
do not use
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
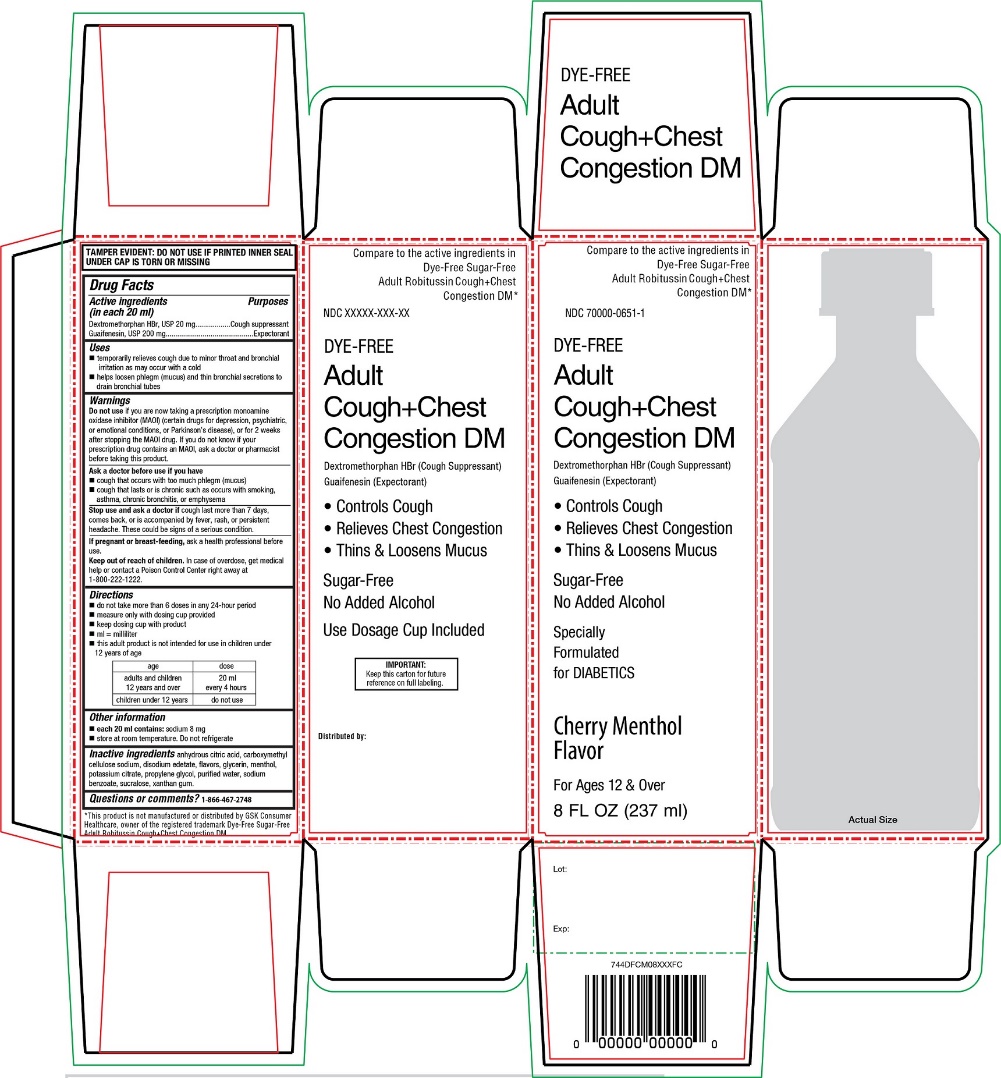
PRINCIPAL DISPLAY PANEL
Compare to the active ingredients in Dye-Free Sugar-Free Adult Robitussin Cough + Chest Congestion DM*
NDC 70000-0651-1
DYE-FREEAdult
Cough+Chest
Congestion DMDextromethorphan HBr (Cough Suppressant)
Guaifenesin (Expectorant)- •
- Controls Cough
- •
- Relieves Chest Congestion
- •
- Thins & Loosens Mucus
Sugar-Free
No Added Alcohol
Specially
Formulated
for DIABETICS
Cherry Menthol FlavorFor Ages 12 & Over
8 FL OZ (237 ml)*This product is not manufactured or distributed by GSK Consumer Healthcare, owner of the registered trademark DYE-FREE Sugar-Free Adult Robitussin Cough+chest Congestion DM.
Distributed by:
-
INGREDIENTS AND APPEARANCE
DYE FREE ADULT COUGH PLUS CHEST CONGESTION DM
dextromethorphan hydrobromide and guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0651 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) POTASSIUM CITRATE (UNII: EE90ONI6FF) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0651-1 1 in 1 CARTON 04/01/2024 1 237 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/01/2024 Labeler - CARDINAL HEALTH (063997360)