Label: HYDRALAZINE HYDROCHLORIDE injection
- NDC Code(s): 70518-3659-0, 70518-3659-1, 70518-3659-2
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 63323-614
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only
-
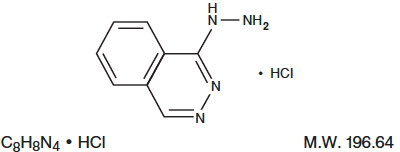
DESCRIPTION:Hydralazine Hydrochloride Injection, USP is an antihypertensive available in a 2 mL vial for intravenous and intramuscular administration. Each mL of the sterile, nonpyrogenic colorless solution ...
-
CLINICAL PHARMACOLOGY:Although the precise mechanism of action of hydralazine is not fully understood, the major effects are on the cardiovascular system. Hydralazine apparently lowers blood pressure by exerting a ...
-
INDICATIONS AND USAGE:Severe essential hypertension when the drug cannot be given orally or when there is an urgent need to lower blood pressure.
-
CONTRAINDICATIONS:Hypersensitivity to hydralazine, coronary artery disease, mitral valvular rheumatic heart disease.
-
WARNINGS:In a few patients, hydralazine may produce a clinical picture simulating systemic lupus erythematosus including glomerulonephritis. In such patients, hydralazine should be discontinued unless the ...
-
PRECAUTIONS:General - Myocardial stimulation produced by hydralazine can cause anginal attacks and ECG changes of myocardial ischemia. The drug has been implicated in the production of myocardial ...
-
ADVERSE REACTIONSTo report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. Adverse reactions with hydralazine hydrochloride are ...
-
OVERDOSAGE:Acute Toxicity - No deaths due to acute poisoning have been reported. Highest known dose survived: adults, 10 g orally. Oral LD - 50in rats: 173 and 187 mg/kg. Signs and ...
-
DOSAGE AND ADMINISTRATION:When there is urgent need, therapy in the hospitalized patient may be initiated intramuscularly or as a rapid intravenous bolus injection directly into the vein. Hydralazine Hydrochloride ...
-
HOW SUPPLIED:20 mg per mL, 1 mL Single Dose Vial - NDC: 70518-3659-00 - NDC: 70518-3659-01 - NDC: 70518-3659-02 - PACKAGING: 25 in 1 TRAY - PACKAGING: 25 in 1 TRAY - PACKAGING: 1 mL in 1 VIAL, SINGLE DOSE - Store at 20° to ...
-
SPL UNCLASSIFIED SECTIONRepackaged and Distributed By: Remedy Repack, Inc. 625 Kolter Dr. Suite #4 Indiana, PA 1-724-465-8762
-
PRINCIPAL DISPLAY PANELDRUG: hydrALAZINE Hydrochloride - GENERIC: hydrALAZINE Hydrochloride - DOSAGE: INJECTION - ADMINSTRATION: INTRAVENOUS - NDC: 70518-3659-0 - NDC: 70518-3659-1 - NDC: 70518-3659-2 - PACKAGING: 1 mL in 1 VIAL ...
-
INGREDIENTS AND APPEARANCEProduct Information