Label: PRIMIDONE tablet
- NDC Code(s): 80005-117-11, 80005-117-14, 80005-117-15, 80005-118-11, view more
- Packager: Carnegie Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
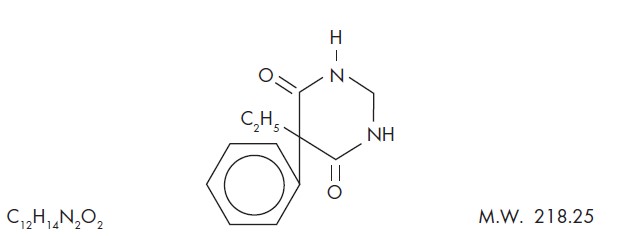
DESCRIPTIONChemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula: Primidone is a white, crystalline, highly stable substance, M.P. 279-284°C. It is poorly soluble in water ...
-
ACTIONSPrimidone tablets raises electro-or chemoshock seizure thresholds or alters seizure patterns in experimental animals. The mechanism(s) of primidone’s antiepileptic action is not known. Primidone ...
-
INDICATIONS AND USAGEPrimidone tablets, used alone or concomitantly with other anticonvulsants, is indicated in the control of grand mal, psychomotor, and focal epileptic seizures. It may control grand mal seizures ...
-
CONTRAINDICATIONSPrimidone is contraindicated in: 1) patients with porphyria and 2) patients who are hypersensitive to phenobarbital (see ACTIONS).
-
WARNINGSThe abrupt withdrawal of antiepileptic medication may precipitate status epilepticus. The therapeutic efficacy of a dosage regimen takes several weeks before it can be assessed. Suicidal Behavior ...
-
PRECAUTIONSThe total daily dosage should not exceed 2 g. Since primidone tablets therapy generally extends over prolonged periods, a complete blood count and a sequential multiple analysis-12 (SMA-12) test ...
-
ADVERSE REACTIONSThe most frequently occurring early side effects are ataxia and vertigo. These tend to disappear with continued therapy, or with reduction of initial dosage. Occasionally, the following have been ...
-
DOSAGE AND ADMINISTRATIONUsual Dosage - Patients 8 years of age and older who have received no previous treatment may be started on primidone tablets according to the following regimen using either 50 mg or scored 250 mg ...
-
HOW SUPPLIED
Primidone Tablets, USP - 50 mg: White, round, flat-faced, bevel-edged tablets, debossed “CP” above “11” on one side and bisected on the other side, in bottles of 100 (NDC 80005-117-11), 500 (NDC ...
-
MEDICATION GUIDEMEDICATION GUIDE - Primidone (“Prim’i done”) Tablets USP, 50 mg and 250 mg - Read this Medication Guide before you start taking Primidone tablets and each time you get a refill. There may be ...
-
PRINCIPAL DISPLAY PANELNDC 80005-117-11 - Primidone Tablets, USP - 50 mg - Each tablet contains: 50 mg of primidone, USP - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx Only - 100 ...
-
PRINCIPAL DISPLAY PANELNDC 80005-118-11 - Primidone Tablets, USP - 250 mg - Each tablet contains: 250 mg of primidone, USP - PHARMACIST: Dispense the accompanying Medication Guide to each patient. Rx Only - 100 ...
-
INGREDIENTS AND APPEARANCEProduct Information