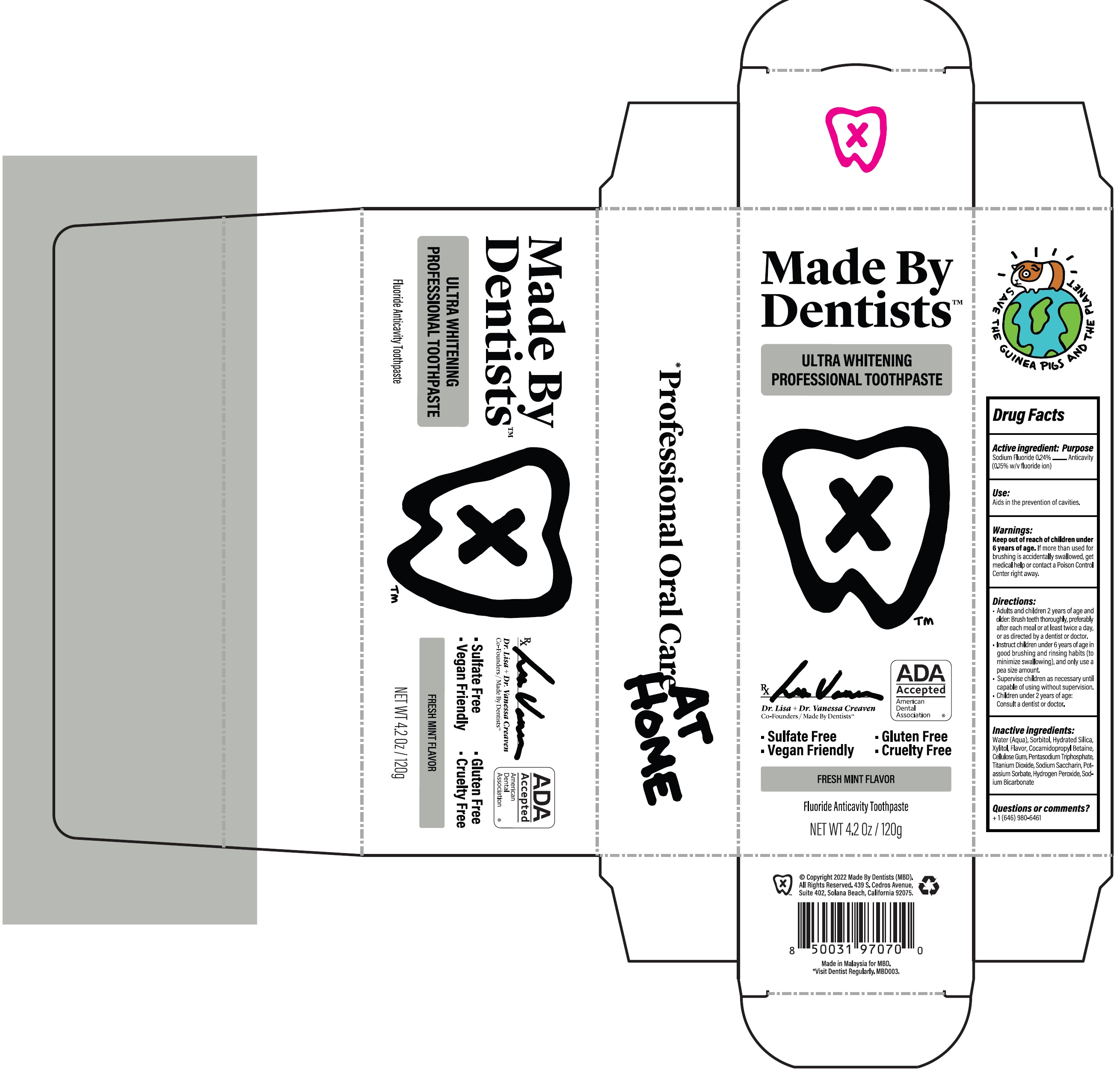
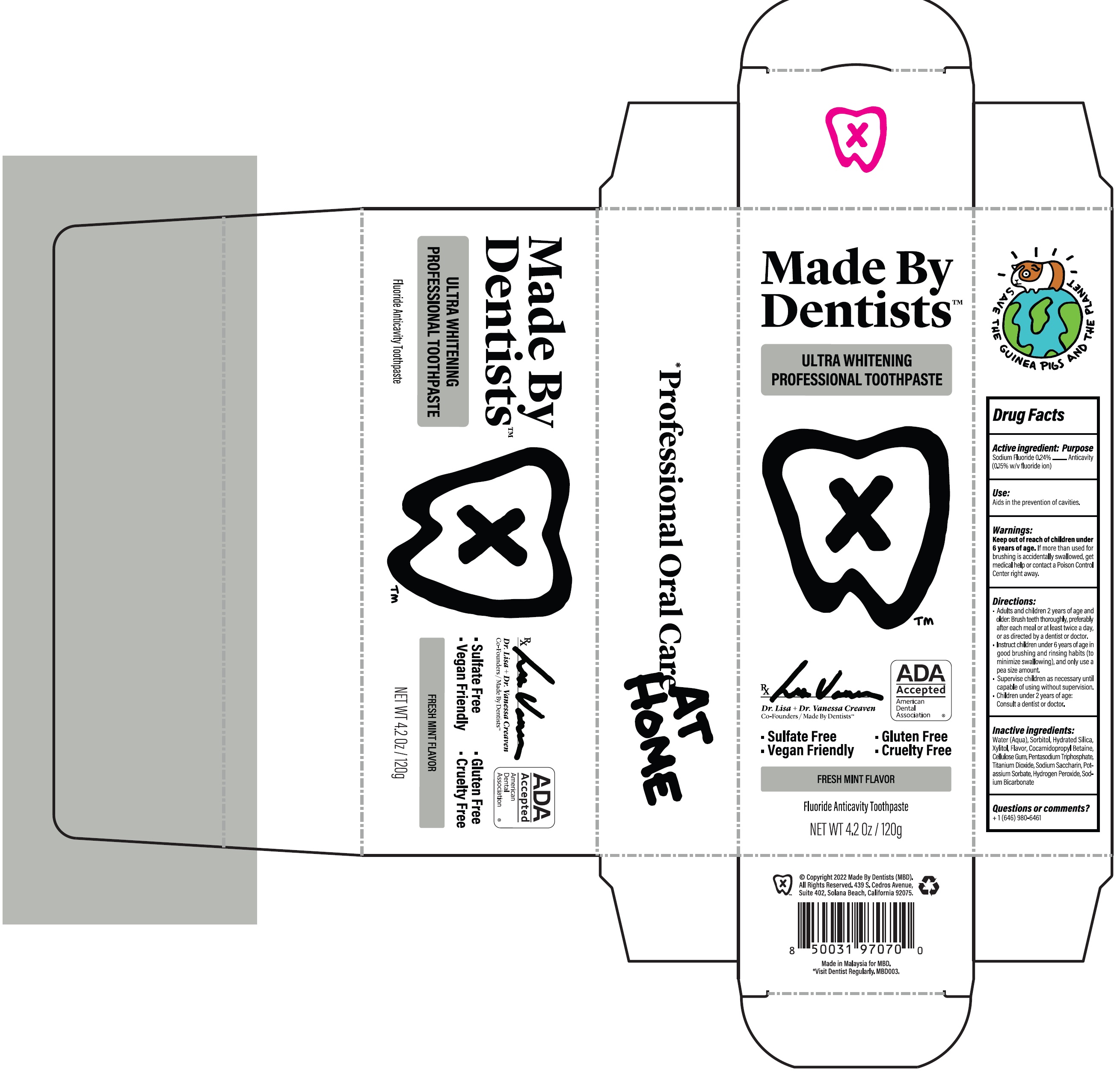
Label: MADE BY DENTISTS ULTRA WHITENING FRESH MINT- sodium fluoride paste
- NDC Code(s): 75065-027-06, 75065-027-07
- Packager: MADE BY DENTISTS INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient:
- Use:
- Warnings:
-
Directions:
- Adults and children 2 years of age and older: Brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or doctor.
- Instruct children under 6 years of age in good brushing and rinsing habits (to minimize swallowing), and only use a pea size amount.
- Supervise children as necessary until capable of using without supervision.
- Children under 2 years of age: Consult a dentist or doctor.
- Inactive ingredients:
- Questions or comments?
- Label 75065-027-06
- Label 75065-027-07
-
INGREDIENTS AND APPEARANCE
MADE BY DENTISTS ULTRA WHITENING FRESH MINT
sodium fluoride pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75065-027 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) HYDRATED SILICA (UNII: Y6O7T4G8P9) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SODIUM TRIPOLYPHOSPHATE ANHYDROUS (UNII: 9SW4PFD2FZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) HYDROGEN PEROXIDE (UNII: BBX060AN9V) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SACCHARIN SODIUM (UNII: SB8ZUX40TY) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75065-027-06 1 in 1 CARTON 02/22/2024 1 120 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:75065-027-07 1 in 1 CARTON 02/22/2024 2 20 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 02/22/2024 Labeler - MADE BY DENTISTS INC (117405870)