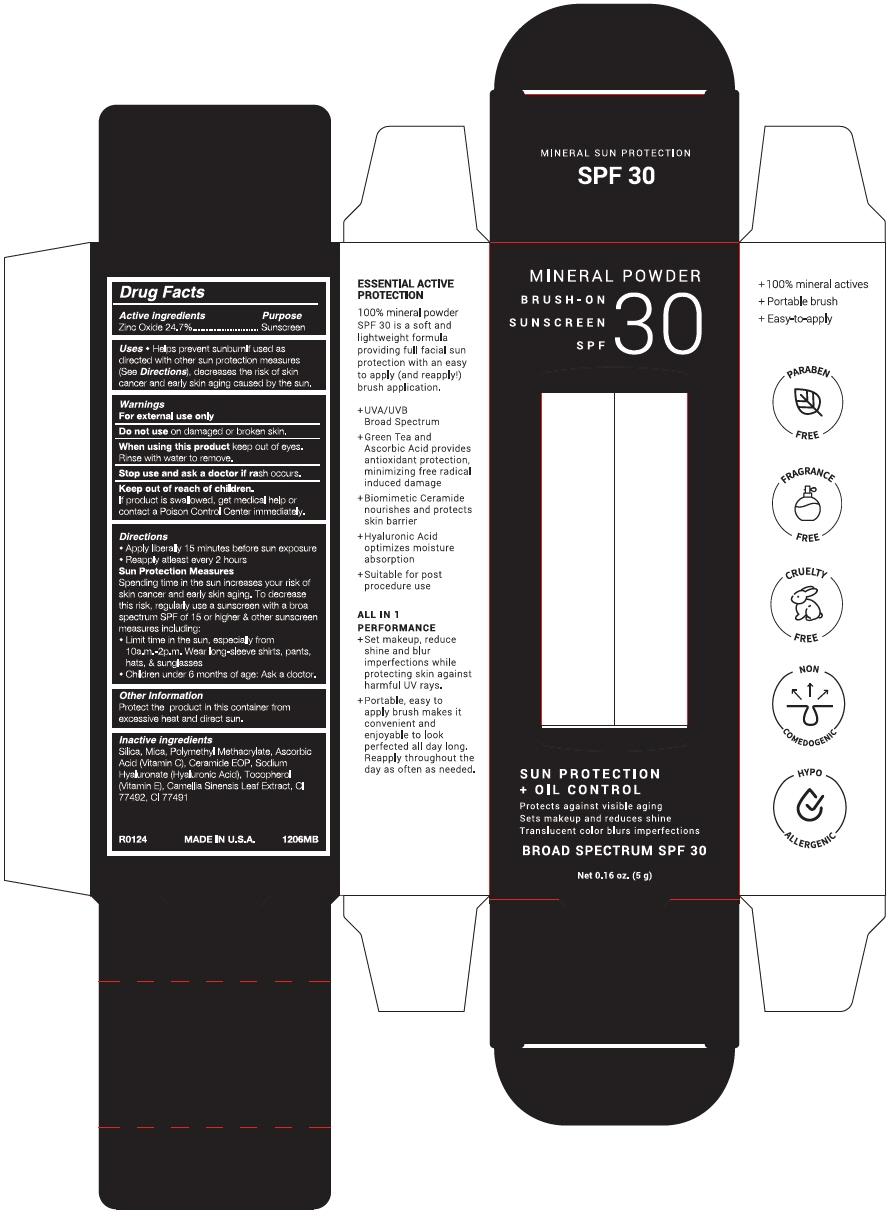
Label: TOPIX MINERAL FINISHING SUNSCREEN SPF 30- zinc oxide powder
- NDC Code(s): 51326-206-01
- Packager: Topiderm, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
-
Uses
- Helps prevent sunburn If used as directed with other sun protection measures (See Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher & other sunscreen measures including:
- Limit time in the sun, especially from 10a.m.-2p.m. Wear long-sleeve shirts, pants, hats, & sunglasses
- Children under 6 months of age: Ask a doctor.
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 5 g Bottle Box
-
INGREDIENTS AND APPEARANCE
TOPIX MINERAL FINISHING SUNSCREEN SPF 30
zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51326-206 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 241 mg in 1 g Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MICA (UNII: V8A1AW0880) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) ASCORBIC ACID (UNII: PQ6CK8PD0R) CERAMIDE 1 (UNII: 5THT33P7X7) HYALURONATE SODIUM (UNII: YSE9PPT4TH) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) GREEN TEA LEAF (UNII: W2ZU1RY8B0) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51326-206-01 1 in 1 BOX 02/26/2024 1 5 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH DRUG M020 02/26/2024 Labeler - Topiderm, Inc (049121643) Registrant - Topiderm, Inc. (049121643) Establishment Name Address ID/FEI Business Operations Aopline Health Industry Technology (Guangzhou) Co., Ltd. 715076108 MANUFACTURE(51326-206)