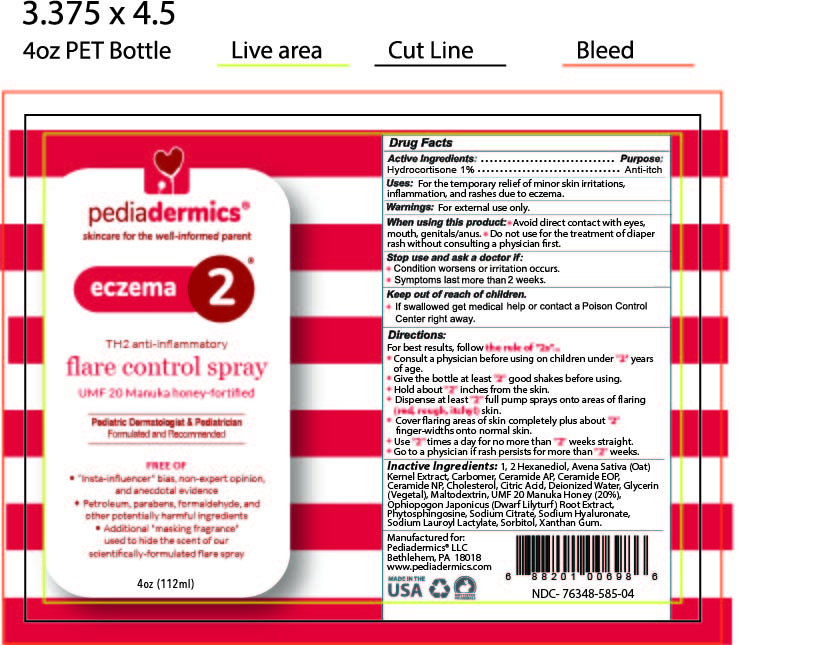
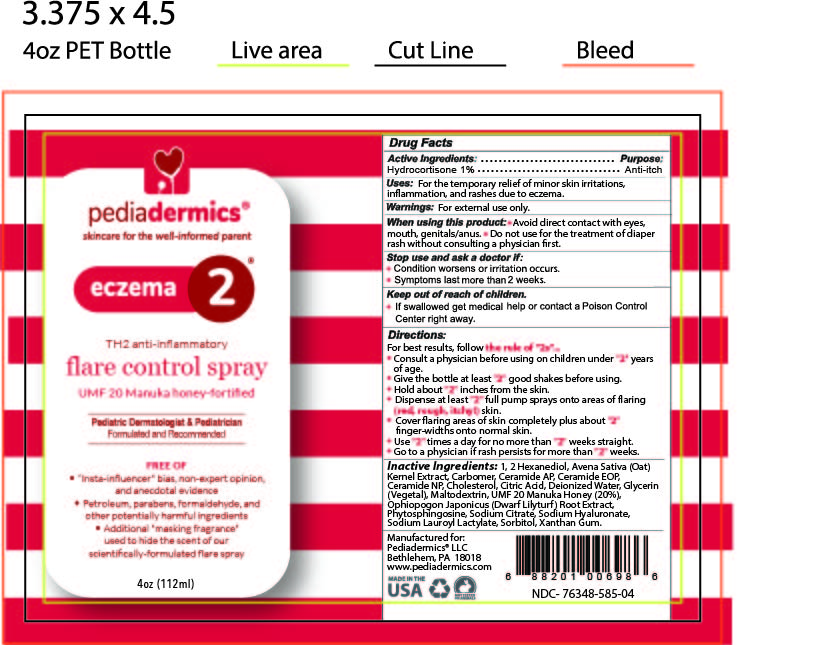
Label: PEDIADERMICS ECZEMA 2- hydrocortisone spray
- NDC Code(s): 76348-585-04
- Packager: RENU LABORATORIES, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITYpediadermics Eczema 2 flare control spray
-
ACTIVE INGREDIENTActive Ingredients: Hydrocortisone 1%
-
PURPOSEPurpose - Anti-itch
-
INDICATIONS & USAGEUses: For the temporary relief of minor skin irritations, inflammation and rashes due to eczema
-
WARNINGSWarnings For external use only
-
WHEN USINGWhen using this product - Avoid direct contact with eyes, mouth, genitals/anus - Do not use for treatment of diaper rash without consulting a physician first
-
STOP USEStop use and ask a doctor if - Condition worsens or irritation occurs - Symptoms last more than 2 weeks
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children - If swallowed get medical help or contact a Poison Control Center right away
-
DOSAGE & ADMINISTRATIONDirections - For best results follow - the rule of "2s" ... Consult a physician before using on children under "2" years of age - Give the bottle at least "2" good shakes before using - Hold the ...
-
INACTIVE INGREDIENTInactive Ingredients - 1, 2 Hexanediol, Avena Sativa (Oat) Kernel Extract, Carbomer, Ceramide AP, Ceramide EOP, Ceramide NP, Cholesterol, Citric Acid, Deionized Water, Glycerin (Vegetal) ...
-
STATEMENT OF IDENTITYManufactured for Pediadermics LLC - Bethlehem PA 18018 - www.pediadermics.com
-
PRINCIPAL DISPLAY PANELBOTTLE ARTWORK
-
INGREDIENTS AND APPEARANCEProduct Information