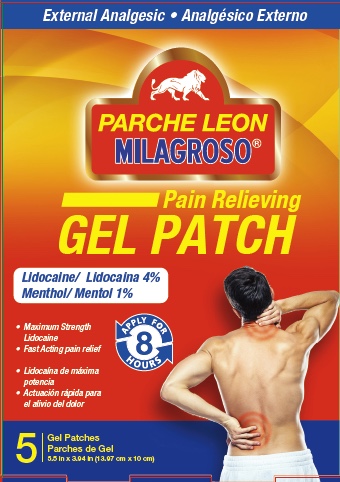
Label: PARCHE DE LEON- lidocaine, menthol patch
- NDC Code(s): 55758-040-01, 55758-040-05
- Packager: Pharmadel LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
-
Warnings
FOR EXTERNAL USE ONLY. Avoid contact with the eyes.
Do not use
- in large quantities, particularly over raw surfaces or blistered areas
- on sensitive skin
- if allergic to any ingredients in this product
-
Directions
- adults & children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: consult a doctor
- clean dry affected area
- remove patch from film
- apply sticky side of patch to affected area
- use one patch at a time
- leave patch on affected area for up to 8-hours at a time
- Other Information
- Inactive Ingredients
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PARCHE DE LEON
lidocaine, menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55758-040 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) DIHYDROXYALUMINUM AMINOACETATE ANHYDROUS (UNII: 1K713C615K) POVIDONE K90 (UNII: RDH86HJV5Z) KAOLIN (UNII: 24H4NWX5CO) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYACRYLIC ACID (800000 MW) (UNII: D0I6NSZ87U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) TARTARIC ACID (UNII: W4888I119H) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55758-040-05 5 in 1 CARTON 03/18/2022 1 NDC:55758-040-01 1 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:55758-040-01 1 in 1 POUCH; Type 0: Not a Combination Product 03/18/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/18/2022 Labeler - Pharmadel LLC (030129680)