Label: TENOFOVIR DISOPROXIL FUMARATE tablet, film coated
- NDC Code(s): 0904-6821-04
- Packager: Major Pharmaceuticals
- This is a repackaged label.
- Source NDC Code(s): 33342-096
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TENOFOVIR DISOPROXIL FUMARATE TABLETS safely and effectively. See full prescribing information for TENOFOVIR DISOPROXIL FUMARATE ...These highlights do not include all the information needed to use TENOFOVIR DISOPROXIL FUMARATE TABLETS safely and effectively. See full prescribing information for TENOFOVIR DISOPROXIL FUMARATE TABLETS.
TENOFOVIR DISOPROXIL FUMARATE tablets, for oral use
Initial U.S. Approval: 2001
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including tenofovir disoproxil fumarate tablets. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in HBV-infected patients who discontinue anti-hepatitis B therapy, including tenofovir disoproxil fumarate tablets. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Tenofovir disoproxil fumarate tablets are a nucleotide analog HIV-1 reverse transcriptase inhibitor and an HBV reverse transcriptase inhibitor and is indicated:
• in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients 2 years of age and older weighing at least 10 kg. (1.1)
• for the treatment of chronic hepatitis B in adults and pediatric patients 2 years and older weighing at least 10 kg.(1.2)DOSAGE AND ADMINISTRATION
• Testing: Prior to or when initiating tenofovir disoproxil fumarate tablets test for hepatitis B virus infection and HIV-1 infection. Prior to initiation and during use of tenofovir disoproxil fumarate tablets, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorous. (2.1)
• Recommended tablet dosage in adults and pediatric patients weighing at least 35 kg: One tenofovir disoproxil fumarate 300 mg tablet once daily taken orally without regard to food. (2.2)
• Recommended dosage in pediatric patients at least 2 years of age and adults:
o Tablets: For patients weighing at least 17 kg who can swallow an intact tablet, one tenofovir disoproxil fumarate tablet (300 mg based on body weight) once daily taken orally without regard to food. (2.2)
• Recommended dosage in renally impaired adult patients:
o Creatinine clearance (CrCl) 30−49 mL/min: 300 mg every 48 hours. (2.4)
o CrCl 10−29 mL/min: 300 mg every 72 to 96 hours. (2.4)
o Hemodialysis: 300 mg every 7 days or after approximately 12 hours of dialysis. (2.4)DOSAGE FORMS AND STRENGTHS
•Tablets: 300 mg of tenofovir disoproxil fumarate. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
• New onset or worsening renal impairment: Can include acute renal failure and Fanconi syndrome. Avoid administering tenofovir disoproxil fumarate tablets with concurrent or recent use of nephrotoxic drugs. (5.2)
• HIV testing: HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with tenofovir disoproxil fumarate tablets. Tenofovir disoproxil fumarate tablets should only be used as part of an appropriate antiretroviral combination regimen in HIV-infected patients with or without HBV coinfection. (5.3)
• Immune reconstitution syndrome: May necessitate further evaluation and treatment. (5.4)
• Decreases in bone mineral density (BMD): Consider assessment of BMD in patients with a history of pathologic fracture or other risk factors for osteoporosis or bone loss. (5.5)
• Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. (5.6)ADVERSE REACTIONS
- •
- In HIV-infected adult subjects: Most common adverse reactions (incidence greater than or equal to 10%, Grades 2–4) were rash, diarrhea, nausea, headache, pain, depression, and asthenia. (6.1)
- •
- In HBV-infected subjects with compensated liver disease: Most common adverse reaction (all grades) was nausea (9%). (6.1)
- •
- In HBV-infected subjects with decompensated liver disease: Most common adverse reactions (incidence greater than or equal to 10%, all grades) were abdominal pain, nausea, insomnia, pruritus, vomiting, dizziness, and pyrexia. (6.1)
- •
- In pediatric subjects: Adverse reactions in pediatric subjects were consistent with those observed in adults. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Macleods Pharma USA,Inc. at 1-888-943-3210 or 1-855-926-3384 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
• Tenofovir disoproxil fumarate increases didanosine concentrations. Dose reduction and close monitoring for didanosine toxicity are warranted. (7.2)
• Coadministration decreases atazanavir concentrations. When coadministered with tenofovir disoproxil fumarate, use atazanavir given with ritonavir. (7.2)
• Coadministration of tenofovir disoproxil fumarate with certain HIV-1 protease inhibitors or certain drugs to treat HCV increases tenofovir concentrations. Monitor for evidence of tenofovir toxicity. (7.2)
• Consult Full Prescribing Information prior to and during treatment for important drug interactions. (7.2)USE IN SPECIFIC POPULATIONS
Lactation: Breastfeeding in HIV-1 infected mothers is not recommended due to the potential for HIV-1 transmission. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
1 INDICATIONS & USAGE
1.1 HIV-1 Infection
1.2 Chronic Hepatitis B
2 DOSAGE & ADMINISTRATION
2.1 Testing Prior to Initiation of Tenofovir Disoproxil Fumarate Tablets for Treatment of HIV-1 Infection or Chronic Hepatitis B
2.2 Recommended Tablet Dosage in Adults and Pediatric Patients 2 Years and Older Weighing at Least 17 kg
2.4 Dosage Adjustment in Patients with Renal Impairment
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection
5.2 New Onset or Worsening Renal Impairment
5.3 Patients Coinfected with HIV-1 and HBV
5.4 Immune Reconstitution Syndrome
5.5 Bone Loss and Mineralization Defects
5.6 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.7 Risk of Adverse Reactions Due to Drug Interactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Affecting Renal Function
7.2 Established and Significant Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Overview of Clinical Trials
14.2 Clinical Trial Results in Adults with HIV-1 Infection
14.3 Clinical Trial Results in Pediatric Subjects with HIV-1 Infection
14.4 Clinical Trial Results in Adults with Chronic Hepatitis B
14.5 Clinical Trial Results in Pediatric Subjects with Chronic Hepatitis B
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: POSTTREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B virus (HBV) have been reported in HBV-infected patients who have discontinued anti-hepatitis B therapy, including tenofovir disoproxil fumarate tablets. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in HBV-infected patients who discontinue anti-hepatitis B therapy, including tenofovir disoproxil fumarate tablets. If appropriate, resumption of anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS & USAGE1.1 HIV-1 Infection - Tenofovir disoproxil fumarate tablets are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1 ...
1.1 HIV-1 Infection
Tenofovir disoproxil fumarate tablets are indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and pediatric patients 2 years of age and older weighing at least 10 kg.
Close1.2 Chronic Hepatitis B
Tenofovir disoproxil fumarate tablets are indicated for the treatment of chronic hepatitis B virus (HBV) in adults and pediatric patients 2 years of age and older weighing at least 10 kg.
-
2 DOSAGE & ADMINISTRATION2.1 Testing Prior to Initiation of Tenofovir Disoproxil Fumarate Tablets for Treatment of HIV-1 Infection or Chronic Hepatitis B - Prior to or when initiating tenofovir disoproxil fumarate ...
2.1 Testing Prior to Initiation of Tenofovir Disoproxil Fumarate Tablets for Treatment of HIV-1 Infection or Chronic Hepatitis B
Prior to or when initiating tenofovir disoproxil fumarate tablets, test patients for HBV infection and HIV-1 infection. Tenofovir disoproxil fumarate tablets alone should not be used in patients with HIV-1 infection [see Warnings and Precautions (5.3)].
Prior to initiation and during use of tenofovir disoproxil fumarate tablets, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.2)].2.2 Recommended Tablet Dosage in Adults and Pediatric Patients 2 Years and Older Weighing at Least 17 kg
The recommended dosage of tenofovir disoproxil fumarate tablets in adults and pediatric patients weighing at least 35 kg is one 300 mg tablet taken orally once daily without regard to food. The dosage for tenofovir disoproxil fumarate tablet is the same for both HIV and HBV indications.
The recommended dosage of tenofovir disoproxil fumarate tablet in adults and pediatric patients 2 years and older weighing at least 17 kg is 8 mg of tenofovir disoproxil fumarate (TDF) per kg of body weight (up to a maximum of 300 mg) once daily. Dosage for pediatric patients 2 years and older weighing between 17 kg and 35 kg and able to swallow an intact tablet is provided in Table 1. Weight should be monitored periodically and the tenofovir disoproxil fumarate tablets dose adjusted accordingly.
Table 1 Recommended Dosing for Patients 2 Years and Older and Weighing at Least 17 kg Using Tenofovir Disoproxil Fumarate TabletsBody Weight (kg)
Dosing of Tenofovir Disoproxil Fumarate Tablets
17 to less than 22
one 150 mg tablet once daily
22 to less than 28
one 200 mg tablet once daily
28 to less than 35
one 250 mg tablet once daily
at least 35
one 300 mg tablet once daily
Close2.4 Dosage Adjustment in Patients with Renal Impairment
Significant increase in drug exposures occurred when tenofovir disoproxil fumarate tablets were administered to subjects with moderate to severe renal impairment (creatinine clearance below 50 mL/min). Table 3 provides dosage interval adjustment for patients with renal impairment. No dosage adjustment of tenofovir disoproxil fumarate tablets 300 mg is necessary for patients with mild renal impairment (creatinine clearance 50–80 mL/min) [see Warnings and Precautions (5.3), Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
Table 3 Dosage Interval Adjustment for Adult Patients with Altered Creatinine Clearance
Creatinine Clearance (mL/min)a
Hemodialysis Patients
50 or greater
30–49
10–29
Every 7 days or after a total of approximately 12 hours of dialysisb
Recommended 300 mg Dosing Interval
Every 24 hours
Every 48 hours
Every 72 to 96 hours
a. Calculated using ideal (lean) body weight.
b. Generally once weekly assuming 3 hemodialysis sessions a week of approximately 4 hours' duration. Tenofovir disoproxil fumarate should be administered following completion of dialysis.
No data are available to make dosage recommendations in patients with creatinine clearance below 10 mL/min who are not on hemodialysis.
No data are available to make dosage recommendations in pediatric patients with renal impairment.
-
3 DOSAGE FORMS & STRENGTHSTenofovir disoproxil fumarate is available as tablets. 300 mg Tablets: 300mg of TDF, which is (equivalent to 245 mg of tenofovir disoproxil). The tablets are blue coloured, oval shaped, biconvex ...
Tenofovir disoproxil fumarate is available as tablets.
300 mg Tablets: 300mg of TDF, which is (equivalent to 245 mg of tenofovir disoproxil). The tablets are blue coloured, oval shaped, biconvex, film coated tablets debossed with 'CL 77' on one side and plain on other side.
Close -
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection - All patients should be tested for the presence of chronic hepatitis B virus (HBV) before or when initiating ...
5.1 Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection
All patients should be tested for the presence of chronic hepatitis B virus (HBV) before or when initiating tenofovir disoproxil fumarate [see Dosage and Administration (2.1)].
Discontinuation of anti-HBV therapy, including tenofovir disoproxil fumarate, may be associated with severe acute exacerbations of hepatitis B. Patients infected with HBV who discontinue tenofovir disoproxil fumarate should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, resumption of anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since posttreatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.5.2 New Onset or Worsening Renal Impairment
Tenofovir is principally eliminated by the kidney. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia), has been reported with the use of tenofovir disoproxil fumarate [see Adverse Reactions (6.2)].
Prior to initiation and during use of tenofovir disoproxil fumarate, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus.
Dosing interval adjustment of tenofovir disoproxil fumarate and close monitoring of renal function are recommended in all patients with creatinine clearance below 50 mL/min [see Dosage and Administration (2.4)]. No safety or efficacy data are available in patients with renal impairment who received tenofovir disoproxil fumarate using these dosing guidelines, so the potential benefit of tenofovir disoproxil fumarate therapy should be assessed against the potential risk of renal toxicity.
Tenofovir disoproxil fumarate should be avoided with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple non-steroidal anti-inflammatory drugs [NSAIDs]) [see Drug Interactions (7.1)]. Cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in HIV-infected patients with risk factors for renal dysfunction who appeared stable on TDF. Some patients required hospitalization and renal replacement therapy. Alternatives to NSAIDs should be considered, if needed, in patients at risk for renal dysfunction.
Persistent or worsening bone pain, pain in extremities, fractures and/or muscular pain or weakness may be manifestations of proximal renal tubulopathy and should prompt an evaluation of renal function in patients at risk of renal dysfunction.5.3 Patients Coinfected with HIV-1 and HBV
Due to the risk of development of HIV-1 resistance, tenofovir disoproxil fumarate should only be used in HIV-1 and HBV coinfected patients as part of an appropriate antiretroviral combination regimen.
HIV-1 antibody testing should be offered to all HBV-infected patients before initiating therapy with tenofovir disoproxil fumarate. It is also recommended that all patients with HIV-1 be tested for the presence of chronic hepatitis B before initiating treatment with tenofovir disoproxil fumarate.
5.4 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in HIV-1 infected patients treated with combination antiretroviral therapy, including tenofovir disoproxil fumarate. During the initial phase of combination antiretroviral treatment, HIV-1 infected patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.5 Bone Loss and Mineralization Defects
Bone Mineral Density
In clinical trials in HIV-1 infected adults, tenofovir disoproxil fumarate was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism, suggesting increased bone turnover relative to comparators [see Adverse Reactions (6.1)]. Serum parathyroid hormone levels and 1,25 Vitamin D levels were also higher in subjects receiving tenofovir disoproxil fumarate.
Clinical trials evaluating tenofovir disoproxil fumarate in pediatric subjects were conducted. Under normal circumstances, BMD increases rapidly in pediatric patients. In HIV-1 infected subjects 2 years to less than 18 years of age, bone effects were similar to those observed in adult subjects and suggest increased bone turnover. Total body BMD gain was less in the tenofovir disoproxil fumarate -treated HIV-1 infected pediatric subjects as compared to the control groups. Similar trends were observed in chronic HBV-infected pediatric subjects 2 years to less than 18 years of age. In all pediatric trials, normal skeletal growth (height) was not affected for the duration of the clinical trials [see Adverse Reactions (6.1)].
The effects of tenofovir disoproxil fumarate -associated changes in BMD and biochemical markers on long-term bone health and future fracture risk in adults and pediatric subjects 2 years and older are unknown. The long-term effect of lower spine and total body BMD on skeletal growth in pediatric patients, and in particular, the effects of long-duration exposure in younger children is unknown.
Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial. Assessment of BMD should be considered for adult and pediatric patients who have a history of pathologic bone fracture or other risk factors for osteoporosis or bone loss. If bone abnormalities are suspected, appropriate consultation should be obtained.Mineralization Defects
Cases of osteomalacia associated with proximal renal tubulopathy, manifested as bone pain or pain in extremities and which may contribute to fractures, have been reported in association with tenofovir disoproxil fumarate use [see Adverse Reactions (6.2)]. Arthralgia and muscle pain or weakness have also been reported in cases of proximal renal tubulopathy. Hypophosphatemia and osteomalacia secondary to proximal renal tubulopathy should be considered in patients at risk of renal dysfunction who present with persistent or worsening bone or muscle symptoms while receiving TDF-containing products [see Warnings and Precautions (5.2)].
5.6 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including TDF, alone or in combination with other antiretrovirals. Treatment with tenofovir disoproxil fumarate should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
Close5.7 Risk of Adverse Reactions Due to Drug Interactions
The concomitant use of tenofovir disoproxil fumarate and other drugs may result in known or potentially significant drug interactions, some of which may lead to possible clinically significant adverse reactions from greater exposures of concomitant drugs [see Drug Interactions (7.2)].
See Table 12 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during therapy with tenofovir disoproxil fumarate; review concomitant medications during therapy with tenofovir disoproxil fumarate; and monitor for adverse reactions associated with the concomitant drugs. -
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: • Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection [see Warnings and Precautions (5.1)] ...
The following adverse reactions are discussed in other sections of the labeling:
• Severe Acute Exacerbation of Hepatitis B in Patients with HBV Infection [see Warnings and Precautions (5.1)].
• New Onset or Worsening Renal Impairment [see Warnings and Precautions (5.2)].
• Immune Reconstitution Syndrome [see Warnings and Precautions (5.4)].
• Bone Loss and Mineralization Defects [see Warnings and Precautions (5.5)].
• Lactic Acidosis/Severe Hepatomegaly with Steatosis [see Warnings and Precautions (5.6)].6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials Experience in HIV-1 Infected Adults
More than 12,000 subjects have been treated with tenofovir disoproxil fumarate alone or in combination with other antiretroviral medicinal products for periods of 28 days to 215 weeks in clinical trials and expanded access programs. A total of 1,544 subjects have received tenofovir disoproxil fumarate 300 mg once daily in clinical trials; over 11,000 subjects have received tenofovir disoproxil fumarate in expanded access programs.
The most common adverse reactions (incidence greater than or equal to 10%, Grades 2–4) identified from any of the 3 large controlled clinical trials include rash, diarrhea, headache, pain, depression, asthenia, and nausea.
Clinical Trials in Treatment-Naïve HIV-1 Infected Adult Subjects
In Trial 903, 600 antiretroviral-naïve subjects received tenofovir disoproxil fumarate (N=299) or stavudine (d4T) (N=301) administered in combination with lamivudine (3TC) and efavirenz (EFV) for 144 weeks. The most common adverse reactions were mild to moderate gastrointestinal events and dizziness. Mild adverse reactions (Grade 1) were common with a similar incidence in both arms and included dizziness, diarrhea, and nausea. Table 4 provides the treatment-emergent adverse reactions (Grades 2−4) occurring in greater than or equal to 5% of subjects treated in any treatment group.
Table 4 Selected Adverse Reactionsa (Grades 2–4) Reported in ≥5% in Any Treatment Group in Trial 903 (0–144 Weeks)
Tenofovir Disoproxil Fumarate + 3TC + EFV
d4T + 3TC + EFV
N=299
N=301
Rash eventb
18%
12%
Headache
14%
17%
Pain
13%
12%
Diarrhea
11%
13%
Depression
11%
10%
Back pain
9%
8%
Nausea
8%
9%
Fever
8%
7%
Abdominal pain
7%
12%
Asthenia
6%
7%
Anxiety
6%
6%
Vomiting
5%
9%
Insomnia
5%
8%
Arthralgia
5%
7%
Pneumonia
5%
5%
Dyspepsia
4%
5%
Dizziness
3%
6%
Myalgia
3%
5%
Lipodystrophyc
1%
8%
Peripheral neuropathyd
1%
5%
a. Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
b. Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, and pustular rash.
c. Lipodystrophy represents a variety of investigator-described adverse events not a protocol-defined syndrome.
d. Peripheral neuropathy includes peripheral neuritis and neuropathy.
Laboratory Abnormalities: Table 5 provides a list of laboratory abnormalities (Grades 3−4) observed in Trial 903. With the exception of fasting cholesterol and fasting triglyceride elevations that were more common in the d4T group (40% and 9%) compared with the tenofovir disoproxil fumarate group (19% and 1%), respectively, laboratory abnormalities observed in this trial occurred with similar frequency in the tenofovir disoproxil fumarate and d4T treatment arms.
Table 5 Grades 3–4 Laboratory Abnormalities Reported in ≥1% of Tenofovir Disoproxil Fumarate-Treated Subjects in Trial 903 (0-144 Weeks)
Tenofovir Disoproxil Fumarate + 3TC + EFV
d4T + 3TC + EFV
N=299
N=301
Any ≥ Grade 3 Laboratory Abnormality
36%
42%
Fasting Cholesterol (>240 mg/dL)
19%
40%
Creatine Kinase (M: >990 U/L; F: >845 U/L)
12%
12%
Serum Amylase (>175 U/L)
9%
8%
AST (M: >180 U/L; F: >170 U/L)
5%
7%
ALT (M: >215 U/L; F: >170 U/L)
4%
5%
Hematuria (>100 RBC/HPF)
7%
7%
Neutrophils (<750/mm3)
3%
1%
Fasting Triglycerides (>750 mg/dL)
1%
9%
Changes in Bone Mineral Density: In HIV-1 infected adult subjects in Trial 903, there was a significantly greater mean percentage decrease from baseline in BMD at the lumbar spine in subjects receiving tenofovir disoproxil fumarate + 3TC + EFV (−2.2% ± 3.9) compared with subjects receiving d4T + 3TC + EFV (−1.0% ± 4.6) through 144 weeks. Changes in BMD at the hip were similar between the two treatment groups (−2.8% ± 3.5 in the tenofovir disoproxil fumarate group vs. −2.4% ± 4.5 in the d4T group). In both groups, the majority of the reduction in BMD occurred in the first 24−48 weeks of the trial and this reduction was sustained through Week 144. Twenty-eight percent of tenofovir disoproxil fumarate -treated subjects vs. 21% of d4T-treated subjects lost at least 5% of BMD at the spine or 7% of BMD at the hip. Clinically relevant fractures (excluding fingers and toes) were reported in 4 subjects in the tenofovir disoproxil fumarate group and 6 subjects in the d4T group. In addition, there were significant increases in biochemical markers of bone metabolism (serum bone-specific alkaline phosphatase, serum osteocalcin, serum C telopeptide, and urinary N telopeptide) and higher serum parathyroid hormone levels and 1,25 Vitamin D levels in the tenofovir disoproxil fumarate group relative to the d4T group; however, except for bone-specific alkaline phosphatase, these changes resulted in values that remained within the normal range [see Warnings and Precautions (5.5)].
In Trial 934, 511 antiretroviral-naïve subjects received efavirenz (EFV) administered in combination with either emtricitabine (FTC) + tenofovir disoproxil fumarate (N=257) or zidovudine (AZT)/lamivudine (3TC) (N=254) for 144 weeks. The most common adverse reactions (incidence greater than or equal to 10%, all grades) included diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash. Table 6 provides the treatment-emergent adverse reactions (Grades 2−4) occurring in greater than or equal to 5% of subjects treated in any treatment group.
Table 6 Selected Adverse Reactionsa (Grades 2−4) Reported in ≥5% in Any Treatment Group in Trial 934 (0−144 Weeks)
Tenofovir Disoproxil Fumarate b + FTC + EFV
AZT/3TC + EFV
N=257
N=254
Fatigue
9%
8%
Depression
9%
7%
Nausea
9%
7%
Diarrhea
9%
5%
Dizziness
8%
7%
Upper respiratory tract infections
8%
5%
Sinusitis
8%
4%
Rash eventc
7%
9%
Headache
6%
5%
Insomnia
5%
7%
Nasopharyngitis
5%
3%
Vomiting
2%
5%
a. Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
b. From Weeks 96 to 144 of the trial, subjects received TRUVADA with EFV in place of tenofovir disoproxil fumarate+ FTC with EFV.
c. Rash event includes rash, exfoliative rash, rash generalized, rash macular, rash maculopapular, rash pruritic, and rash vesicular.
Laboratory Abnormalities: Laboratory abnormalities observed in this trial were generally consistent with those seen in previous trials (Table 7).
Table 7 Significant Laboratory Abnormalities Reported in ≥1% of Subjects in Any Treatment Group in Trial 934 (0-144 Weeks)
Tenofovir disoproxil fumarate + FTC + EFVa
AZT/3TC + EFV
N=257
N=254
Any ≥ Grade 3 Laboratory Abnormality
30%
26%
Fasting Cholesterol (>240 mg/dL)
22%
24%
Creatine Kinase (M: >990 U/L; F: >845 U/L)
9%
7%
Serum Amylase (>175 U/L)
8%
4%
Alkaline Phosphatase (>550 U/L)
1%
0%
AST (M: >180 U/L; F: >170 U/L)
3%
3%
ALT (M: >215 U/L; F: >170 U/L)
2%
3%
Hemoglobin (<8.0 mg/dL)
0%
4%
Hyperglycemia (>250 mg/dL)
2%
1%
Hematuria (>75 RBC/HPF)
3%
2%
Glycosuria (≥3+)
<1%
1%
Neutrophils (<750/mm3)
3%
5%
Fasting Triglycerides (>750 mg/dL)
4%
2%
a. From Weeks 96 to 144 of the trial, subjects received TRUVADA with EFV in place of tenofovir disoproxil fumarate + FTC with EFV.
Clinical Trials in Treatment-Experienced HIV-1 Infected Adult Subjects
In Trial 907, the adverse reactions seen in HIV-1 infected treatment-experienced subjects were generally consistent with those seen in treatment-naïve subjects, including mild to moderate gastrointestinal events, such as nausea, diarrhea, vomiting, and flatulence. Less than 1% of subjects discontinued participation in the clinical trials due to gastrointestinal adverse reactions. Table 8 provides the treatment-emergent adverse reactions (Grades 2−4) occurring in greater than or equal to 3% of subjects treated in any treatment group.
Table 8 Selected Adverse Reactions a (Grades 2-4) Reported in ≥3% in Any Treatment Group in Trial 907 (0-48 Weeks)
Tenofovir Disoproxil Fumarate N=368 (Week 0-24)
Placebo N=182 (Week 0-24)
Tenofovir Disoproxil Fumarate N=368 (Week 0-48)
Placebo Crossover to Tenofovir Disoproxil Fumarate N=170 (Week 24-48)
Body as a Whole
Asthenia
7%
6%
11%
1%
Pain
7%
7%
12%
4%
Headache
5%
5%
8%
2%
Abdominal pain
4%
3%
7%
6%
Back pain
3%
3%
4%
2%
Chest pain
3%
1%
3%
2%
Fever
2%
2%
4%
2%
Digestive System
Diarrhea
11%
10%
16%
11%
Nausea
8%
5%
11%
7%
Vomiting
4%
1%
7%
5%
Anorexia
3%
2%
4%
1%
Dyspepsia
3%
2%
4%
2%
Flatulence
3%
1%
4%
1%
Respiratory
Pneumonia
2%
0%
3%
2%
Nervous System
Depression
4%
3%
8%
4%
Insomnia
3%
2%
4%
4%
Peripheral neuropathyb
3%
3%
5%
2%
Dizziness
1%
3%
3%
1%
Skin and Appendage
Rash eventc
5%
4%
7%
1%
Sweating
3%
2%
3%
1%
Musculoskeletal
Myalgia
3%
3%
4%
1%
Metabolic
Weight loss
2%
1%
4%
2%
a. Frequencies of adverse reactions are based on all treatment-emergent adverse events, regardless of relationship to study drug.
b. Peripheral neuropathy includes peripheral neuritis and neuropathy.
c. Rash event includes rash, pruritus, maculopapular rash, urticaria, vesiculobullous rash, and pustular rash.
Laboratory Abnormalities: Table 9 provides a list of Grade 3−4 laboratory abnormalities observed in Trial 907. Laboratory abnormalities occurred with similar frequency in the tenofovir disoproxil fumarate and placebo groups.
Table 9 Grades 3–4 Laboratory Abnormalities Reported in ≥1% of Tenofovir Disoproxil Fumarate Tablets-Treated Subjects in Trial 907 (0-48 Weeks)Tenofovir Disoproxil Fumarate N=368 (Week 0-24)
Placebo N=182 (Week 0-24)
Tenofovir Disoproxil Fumarate N=368 (Week 0-48)
Placebo Crossover to
Tenofovir Disoproxil Fumarate N=170 (Week 24-48)
Any ≥ Grade 3 Laboratory Abnormality
25%
38%
35%
34%
Triglycerides (>750 mg/dL)
8%
13%
11%
9%
Creatine Kinase (M: >990 U/L; F: >845 U/L)
7%
14%
12%
12%
Serum Amylase (>175 U/L)
6%
7%
7%
6%
Glycosuria (≥3+)
3%
3%
3%
2%
AST (M: >180 U/L; F: >170 U/L)
3%
3%
4%
5%
ALT (M: >215 U/L; F: >170 U/L)
2%
2%
4%
5%
Serum Glucose (>250 U/L)
2%
4%
3%
3%
Neutrophils (<750/mm3)
1%
1%
2%
1%
Adverse Reactions from Clinical Trials Experience in HIV-1 Infected Pediatric Subjects 2 Years and Older
Assessment of adverse reactions is based on two randomized trials (Trials 352 and 321) in 184 HIV-1 infected pediatric subjects (2 years to less than 18 years of age) who received treatment with tenofovir disoproxil fumarate (N=93) or placebo/active comparator (N=91) in combination with other antiretroviral agents for 48 weeks [see Clinical Studies (14.3)]. The adverse reactions observed in subjects who received treatment with tenofovir disoproxil fumarate were consistent with those observed in clinical trials in adults.In Trial 352, 89 pediatric subjects (2 years to less than 12 years of age) received tenofovir disoproxil fumarate for a median exposure of 104 weeks. Of these, 4 subjects discontinued from the trial due to adverse reactions consistent with proximal renal tubulopathy. Three of these 4 subjects presented with hypophosphatemia and also had decreases in total body or spine BMD Z-score [see Warnings and Precautions (5.5)].
Changes in Bone Mineral Density: In Trial 321 (12 years to less than 18 years of age), the mean rate of BMD gain at Week 48 was less in the tenofovir disoproxil fumarate group compared to the placebo group. Six tenofovir disoproxil fumarate-treated subjects and one placebo-treated subject had significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline BMD Z-scores were −0.341 for lumbar spine and −0.458 for total body in the 28 subjects who were treated with tenofovir disoproxil fumarate for 96 weeks. In Trial 352 (2 years to less than 12 years of age), the mean rate of BMD gain in lumbar spine at Week 48 was similar between the Tenofovir disoproxil fumarate and the d4T or AZT treatment groups. Total body BMD gain was less in the tenofovir disoproxil fumarate group compared to the d4T or AZT treatment group. One tenofovir disoproxil fumarate -treated subject and none of the d4T-or AZT-treated subjects experienced significant (greater than 4%) lumbar spine BMD loss at Week 48. Changes from baseline in BMD Z-scores were −0.012 for lumbar spine and −0.338 for total body in the 64 subjects who were treated with tenofovir disoproxil fumarate for 96 weeks. In both trials, skeletal growth (height) appeared to be unaffected for the duration of the clinical trials [see Warnings and Precautions (5.5)].
Adverse Reactions from Clinical Trials Experience in HBV-Infected Adults
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Compensated Liver Disease
In controlled clinical trials in 641 subjects with chronic hepatitis B (0102 and 0103), more subjects treated with tenofovir disoproxil fumarate during the 48-week double-blind period experienced nausea: 9% with tenofovir disoproxil fumarate versus 2% with HEPSERA. Other treatment-emergent adverse reactions reported in more than 5% of subjects treated with tenofovir disoproxil fumarate included: abdominal pain, diarrhea, headache, dizziness, fatigue, nasopharyngitis, back pain, and skin rash.
In Trials 0102 and 0103, during the open-label phase of treatment with tenofovir disoproxil fumarate (weeks 48–384), 2% of subjects (13/585) experienced a confirmed increase in serum creatinine of 0.5 mg/dL from baseline. No significant change in the tolerability profile was observed with continued treatment for up to 384 weeks.
Laboratory Abnormalities: Table 10 provides a list of Grade 3–4 laboratory abnormalities through Week 48. Grades 3–4 laboratory abnormalities were similar in subjects continuing tenofovir disoproxil fumarate treatment for up to 384 weeks in these trials.
Table 10 Grades 3-4 Laboratory Abnormalities Reported in ≥1% of Tenofovir Disoproxil Fumarate-Treated Subjects in Trials 0102 and 0103 (0-48 Weeks)
Tenofovir Disoproxil Fumarate N=426
HEPSERA N=215
Any ≥ Grade 3 Laboratory Abnormality
19%
13%
Creatine Kinase (M: >990 U/L; F: >845 U/L)
2%
3%
Serum Amylase (>175 U/L)
4%
1%
Glycosuria (≥3+)
3%
<1%
AST (M: >180 U/L; F: >170 U/L)
4%
4%
ALT (M: >215 U/L; F: >170 U/L)
10%
6%
The overall incidence of on-treatment ALT flares (defined as serum ALT greater than 2 × baseline and greater than 10 × ULN, with or without associated symptoms) was similar between tenofovir disoproxil fumarate (2.6%) and HEPSERA (2%). ALT flares generally occurred within the first 4 to 8 weeks of treatment and were accompanied by decreases in HBV DNA levels. No subject had evidence of decompensation. ALT flares typically resolved within 4 to 8 weeks without changes in study medication.
The adverse reactions observed in subjects with chronic hepatitis B and lamivudine resistance who received treatment with tenofovir disoproxil fumarate were consistent with those observed in other HBV clinical trials in adults.
Clinical Trials in Adult Subjects with Chronic Hepatitis B and Decompensated Liver Disease
In Trial 0108, a small randomized, double-blind, active-controlled trial, subjects with chronic HBV and decompensated liver disease received treatment with tenofovir disoproxil fumarate or other antiviral drugs for up to 48 weeks [see Clinical Studies (14.4)]. Among the 45 subjects receiving tenofovir disoproxil fumarate, the most frequently reported treatment-emergent adverse reactions of any severity were abdominal pain (22%), nausea (20%), insomnia (18%), pruritus (16%), vomiting (13%), dizziness (13%), and pyrexia (11%). Two of 45 (4%) subjects died through Week 48 of the trial due to progression of liver disease. Three of 45 (7%) subjects discontinued treatment due to an adverse event. Four of 45 (9%) subjects experienced a confirmed increase in serum creatinine of 0.5 mg/dL (1 subject also had a confirmed serum phosphorus less than 2 mg/dL through Week 48). Three of these subjects (each of whom had a Child-Pugh score greater than or equal to 10 and MELD score greater than or equal to 14 at entry) developed renal failure. Because both tenofovir disoproxil fumarate and decompensated liver disease may have an impact on renal function, the contribution of tenofovir disoproxil fumarate to renal impairment in this population is difficult to ascertain.
One of 45 subjects experienced an on-treatment hepatic flare during the 48-week trial.Adverse Reactions from Clinical Trials Experience in HBV-Infected Pediatric Subjects 2 Years and Older
Assessment of adverse reactions in pediatric subjects infected with chronic HBV is based on two randomized trials: Trial GS-US- 174-0115 in 106 subjects (12 years to less than 18 years of age) receiving treatment with tenofovir disoproxil fumarate (N=52) or placebo (N=54) for 72 weeks and Trial GS-US-174- 0144 in 89 subjects (2 years to less than 12 years of age) receiving treatment with tenofovir disoproxil fumarate (N=60) or placebo (N=29) for 48 weeks [see Clinical Studies (14.5)]. The adverse reactions observed in pediatric subjects who received treatment with tenofovir disoproxil fumarate were consistent with those observed in clinical trials of tenofovir disoproxil fumarate in adults.
In Trial 115 (12 years to less than 18 years of age) and Trial 144 (2 years to less than 12 years of age), both the tenofovir disoproxil fumarate and placebo treatment arms experienced an overall increase in mean lumbar spine and total body BMD over 72 and 48 weeks, respectively, as expected for a pediatric population (Table 11). In Trial 115, the mean percentage BMD gains from baseline to Week 72 in lumbar spine and total body BMD in tenofovir disoproxil fumarate -treated subjects were less than the mean percentage BMD gains observed in placebo-treated subjects (Table 11).Three subjects (6%) in the tenofovir disoproxil fumarate group and two subjects (4%) in the placebo group had significant (greater than or equal to 4%) lumbar spine BMD loss at Week 72.In Trial 144 (2 years to less than 12 years of age), mean percentage BMD gains from baseline to Week 48 in lumbar spine and total body BMD in tenofovir disoproxil fumarate -treated subjects were less than the mean percentage BMD gains observed in placebo-treated subjects. At Week 48, the cumulative percentage of subjects with greater than or equal to 4% decreases in spine or whole body BMD was numerically higher for subjects in the TDF group compared with the placebo group (Table 11). As observed in pediatric studies of HIV-infected subjects, normal skeletal growth (height) was not affected for the duration of the clinical trial [see Warnings and Precautions (5.5)].
Table 11 Change in Bone Mineral Density from Baseline in Pediatric Subjects 2 Years to <12 Years of Age (Trials 115 and 144)
Trial 115
(Week 72)
Trial 144
(Week 48)
Tenofovir Disoproxil Fumarate (N=52)
Placebo (N=54)
Tenofovir Disoproxil Fumarate (N=60)
Placebo (N=29)
Mean percentage change in BMD
Lumbar spine
+5%
+8%
+4%
+8%
Total body
+3%
+5%
+5%
+9%
Cumulative incidence of ≥4% decrease in BMD
Lumbar spine
6%
4%
18%
7%
Total body
0%
2%
7%
0%
Baseline BMD Z-score (mean)
Lumbar spine
-0.43
-0.28
+0.02
-0.29
Total body
-0.20
-0.26
+0.11
-0.05
Mean change in BMD Z-score
Lumbar spine
-0.05
+0.07
-0.12
+0.14
Total body
-0.15
+0.06
-0.18
+0.22
The effects of tenofovir disoproxil fumarate -associated changes in BMD and biochemical markers on long-term bone health and future fracture risk in pediatric patients 2 years and older are unknown. The long-term effect of lower spine and total body BMD on skeletal growth in pediatric patients 2 years and older, and in particular, the effects of long-duration exposure in younger children is unknown [see Warnings and Precautions (5.5)].
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of tenofovir disoproxil fumarate. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune System Disorders
allergic reaction, including angioedema
Metabolism and Nutrition Disorders
lactic acidosis, hypokalemia, hypophosphatemia
Respiratory, Thoracic, and Mediastinal Disorders
dyspnea
Gastrointestinal Disorders
pancreatitis, increased amylase, abdominal pain
Hepatobiliary Disorders
hepatic steatosis, hepatitis, increased liver enzymes (most commonly AST, ALT gamma GT)
Skin and Subcutaneous Tissue Disorders
rash
Musculoskeletal and Connective Tissue Disorders
rhabdomyolysis, osteomalacia (manifested as bone pain and which may contribute to fractures), muscular weakness, myopathy
Renal and Urinary Disorders
acute renal failure, renal failure, acute tubular necrosis, Fanconi syndrome, proximal renal tubulopathy, interstitial nephritis (including acute cases), nephrogenic diabetes insipidus, renal insufficiency, increased creatinine, proteinuria, polyuriaGeneral Disorders and Administration Site Conditions
asthenia
The following adverse reactions, listed under the body system headings above, may occur as a consequence of proximal renal tubulopathy: rhabdomyolysis, osteomalacia, hypokalemia, muscular weakness, myopathy, hypophosphatemia. -
7 DRUG INTERACTIONS7.1 Drugs Affecting Renal Function - Tenofovir is primarily eliminated by the kidneys [see Clinical Pharmacology (12.3)]. Coadministration of tenofovir disoproxil fumarate with drugs that ...
7.1 Drugs Affecting Renal Function
Tenofovir is primarily eliminated by the kidneys [see Clinical Pharmacology (12.3)]. Coadministration of tenofovir disoproxil fumarate with drugs that areeliminated by active tubular secretion may increase concentrations oftenofovir and/or the coadministered drug. Some examples include, but arenot limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir,aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.2)]. Drugs that decrease renal function mayincrease concentrations of tenofovir.
In the treatment of chronic hepatitis B, tenofovir disoproxil fumarate should not be administered in combination with HEPSERA (adefovir dipivoxil).
Close7.2 Established and Significant Interactions
Table 12 provides a listing of established or clinically significant drug interactions. The drug interactions described are based on studies conducted with TDF [see Clinical Pharmacology (12.3)].
Table 12 Established and Significanta Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Trials
Concomitant Drug Class: Drug Name
Effect on Concentrationb
Clinical Comment
NRTI:
didanosine
↑ didanosine
Patients receiving tenofovir disoproxil fumarate and didanosine should be monitored closely for didanosine-associated adverse reactions. Discontinue didanosine in patients who develop didanosine-associated adverse reactions. Higher didanosine concentrations could potentiate didanosine-associated adverse reactions, including pancreatitis, and neuropathy. Suppression of CD4+ cell counts has been observed in patients receiving tenofovir disoproxil fumarate with didanosine 400 mg daily.
In patients weighing greater than 60 kg, reduce the didanosine dose to 250 mg when it is coadministered with tenofovir disoproxil fumarate. In patients weighing less than 60 kg, reduce the didanosine dose to 200 mg when it is coadministered with tenofovir disoproxil fumarate. When coadministered, tenofovir disoproxil fumarate and Videx EC may be taken under fasted conditions or with a light meal (less than 400 kcal, 20% fat).
HIV-1 Protease
Inhibitors:
atazanavir
lopinavir/ritonavir
atazanavir/ritonavir
darunavir/ritonavir
↓ atazanavir
↑ tenofovir
When coadministered with tenofovir disoproxil fumarate, atazanavir 300 mg
should be given with ritonavir 100 mg.
Monitor patients receiving tenofovir disoproxil fumarate concomitantly with lopinavir/ritonavir, ritonavir-boosted atazanavir, or ritonavir-
boosted darunavir for TDF-associated adverse
reactions. Discontinue tenofovir disoproxil fumarate in patients who develop TDF-associated adverse reactions.
Hepatitis C Antiviral Agents: sofosbuvir/velpatasvir sofosbuvir/velpatasvir/ voxilaprevir
ledipasvir/sofosbuvir
↑ tenofovir
Monitor patients receiving tenofovir disoproxil fumarate concomitantly with EPCLUSA® (sofosbuvir/velpatasvir) for adverse reactions associated with TDF.
Monitor patients receiving tenofovir disoproxil fumarate concomitantly with HARVONI® (ledipasvir/sofosbuvir) without an HIV-1 protease inhibitor/ritonavir or an HIV-1 protease inhibitor/cobicistat combination, for adverse reactions associated with TDF. In patients receiving tenofovir disoproxil fumarate concomitantly with HARVONI and an HIV-1 protease inhibitor/ritonavir or an HIV-1 protease inhibitor/cobicistat combination, consider an alternative HCV or antiretroviral therapy, as the safety of increased tenofovir concentrations in this setting has not been established. If coadministration is necessary, monitor for adverse reactions associated with TDF.
a. This table is not all inclusive.
b. ↑=Increase, ↓=Decrease -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to tenofovir disoproxil fumarate during pregnancy ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to tenofovir disoproxil fumarate during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
Available data from the APR show no increase in the overall risk of major birth defects with first trimester exposure for tenofovir disoproxil fumarate (TDF) (2.1%) compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data). The rate of miscarriage for individual drugs is not reported in the APR. In the U.S. general population, the estimated background risk of miscarriage in clinically recognized pregnancies is 15–20%.
Published studies in HBV-infected subjects do not report an increased risk of adverse pregnancy-related outcomes with the use of tenofovir disoproxil fumarate during the third trimester of pregnancy (see Data).
In animal reproduction studies, no adverse developmental effects were observed when TDF was administered at doses/exposures ≥14 (TDF) and 2.7 (tenofovir) times those of the recommended daily dose of tenofovir disoproxil fumarate (see Data).Data
Human Data
Based on prospective reports from the APR exposures to TDF-containing regimens during pregnancy resulting in live births (including 3,342 exposed in the first trimester and 1,475 exposed in the second/third trimester), there was no increase in overall major birth defects with TDF compared with the background birth defect rate of 2.7% in a U.S. reference population of the MACDP. The prevalence of major birth defects in live births was 2.3% (95% CI: 1.8% to 2.8%) with first trimester exposure to TDF-containing regimens, and 2.1% (95% CI: 1.4% to 3.0%) with the second/third trimester exposure to TDF-containing regimens.
Prospective reports from the APR of overall major birth defects in pregnancies exposed to TDF are compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations, as well as confounding due to the underlying disease.
In published data from three controlled clinical trials, a total of 327 pregnant women with chronic HBV infection were administered tenofovir disoproxil fumarate from 28 to 32 weeks gestation through 1 to 2 months postpartum and followed for up to 12 months after delivery. There were no new safety findings in pregnant women compared with the known safety profile of tenofovir disoproxil fumarate in HBV-infected adults. An increased risk of adverse pregnancy-related outcomes was not observed; 2 stillbirths were identified, and there was 1 major birth defect (talipes) and 1 occurrence of multiple congenital abnormalities (not further specified) in tenofovir disoproxil fumarate-exposed infants. Infants were followed for up to 12 months after delivery; there were no clinically relevant drug-related safety findings in infants exposed to tenofovir disoproxil fumarate during late gestation.Animal Data
TDF was administered orally to pregnant rats (at 0, 50, 150, or 450 mg/kg/day) and rabbits (at 0, 30, 100, or 300 mg/kg/day) through organogenesis (on gestation days 7 through 17, and 6 through 18, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with TDF in rats at doses up to 14 times the human dose based on body surface area comparisons and in rabbits at doses up to 19 times the human dose based on body surface area comparisons. In a pre/postnatal development study in rats, TDF was administered orally through lactation at doses up to 600 mg/kg/day; no adverse effects were observed in the offspring at tenofovir exposures of approximately 2.7 times higher than human exposures at the recommended daily dose of tenofovir disoproxil fumarate.
8.2 Lactation
Risk Summary
Based on published data, tenofovir has been shown to be present in human breast milk (see Data). It is not known if tenofovir affects milk production or has effects on the breastfed child.
Treatment of HIV-1 infection:
The Centers for Disease Control and Prevention recommend that HIV-1 infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV-1.
Because of the potential for: (1) HIV transmission (in HIV-negative infants); (2) developing viral resistance (in HIV-positive infants); and (3) adverse reactions in a breastfed infant similar to those seen in adults, instruct mothers not to breastfeed if they are taking tenofovir disoproxil fumarate for the treatment of HIV-1.
Treatment of HBV infection:
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for tenofovir disoproxil fumarate and any potential adverse effects on the breastfed infant from tenofovir disoproxil fumarate or from the underlying maternal condition.
Data
In a study of 50 HIV-uninfected, breastfeeding women on a tenofovir-containing regimen initiated between 1 and 24 weeks postpartum (median 13 weeks), tenofovir was undetectable in the plasma of most infants after 7 days of treatment in mothers. There were no serious adverse events in mothers or infants.8.4 Pediatric Use
Pediatric Patients 2 Years and Older with HIV-1 Infection
The safety and effectiveness of tenofovir disoproxil fumarate in pediatric patients 2 years to less than 18 years of age is supported by data from two randomized trials. Trial 352 was a randomized controlled trial in 92 HIV-1 treatment experienced subjects 2 years to less than 12 years of age who were virologically suppressed on a stavudine-or zidovudine-containing regimen and were randomized to either switch to a tenofovir disoproxil fumarate -containing regimen (N=44) or stay on their original regimen (N=48) for 48 weeks. At Week 48, 89% of subjects in the tenofovir disoproxil fumarate treatment group and 90% of subjects in the d4T or AZT treatment group had HIV-1 RNA concentrations <400 copies/mL. Trial 321 was a placebo-controlled trial in 87 HIV-1 treatment experienced subjects 12 years to less than 18 years of age who were treated with tenofovir disoproxil fumarate (N=45) or placebo (N=42) in combination with an optimized background regimen for 48 weeks. Overall, the trial failed to show a difference in virologic response between the tenofovir disoproxil fumarate and placebo groups. Subgroup analyses suggest the lack of difference in virologic response may be attributable to imbalances between treatment arms in baseline viral susceptibility to tenofovir disoproxil fumarate and OBR [see Adverse Reactions (6.1) and Clinical Studies (14.3)].
Although changes in HIV-1 RNA in these highly treatment-experienced subjects in Trial 321 were less than anticipated, the pharmacokinetic profile of tenofovir in patients 2 years to less than 18 years of age at the recommended doses was similar to that found to be safe and effective in adult clinical trials [see Clinical Pharmacology (12.3)].
The effects of tenofovir disoproxil fumarate -associated changes in BMD and biochemical markers on long-term bone health and future fracture risk in HIV-1 pediatric patients 2 years and older are unknown. The long-term effect of lower spine and total body BMD on skeletal growth in pediatric patients 2 years and older, and in particular, the effects of long-duration exposure in younger children is unknown [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].Safety and effectiveness of tenofovir disoproxil fumarate in pediatric patients younger than 2 years of age and weighing less than 10 kg with HIV-1 infection have not been established.
Pediatric Patients 2 Years of Age and Older with Chronic Hepatitis B
The safety and effectiveness of tenofovir disoproxil fumarate in pediatric patients 2 years to less than 18 years of age is supported by data from two randomized trials (Trial 115 and Trial 144) in which tenofovir disoproxil fumarate was administered to HBV-infected treatment-experienced subjects.
In Trial 115, 106 HBeAg negative (9%) and positive (91%) subjects 12 years to less than 18 years of age with chronic HBV infection were randomized to receive blinded treatment with tenofovir disoproxil fumarate or placebo for 72 weeks. At Week 72, 88% of subjects in the tenofovir disoproxil fumarate group and 0% of subjects in the placebo group had HBV DNA <400 copies/mL (69 IU/mL).In Trial 144, 89 HBeAg positive (96%) and negative (4%) subjects 2 years to less than 12 years of age were treated with tenofovir disoproxil fumarate 8 mg/kg up to maximum dose of 300 mg or placebo once daily for 48 weeks. At Week 48, 77% of subjects in the tenofovir disoproxil fumarate group and 7% of subjects in the placebo group had HBV DNA <400 copies/mL (69 IU/mL).
The effects of tenofovir disoproxil fumarate-associated changes in BMD and biochemical markers on long-term bone health and future fracture risk in chronic HBV-infected pediatric patients 2 years and older are unknown. The long-term effect of lower spine and total body BMD on skeletal growth in pediatric patients 2 years and older, and in particular, the effects of long-duration exposure in younger children is unknown [see Warnings and Precautions (5.5), Adverse Reactions (6.1)].Safety and effectiveness of tenofovir disoproxil fumarate in chronic HBV-infected pediatric patients younger than 2 years of age and weighing less than 10 kg have not been established.
8.5 Geriatric Use
Clinical trials of tenofovir disoproxil fumarate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for the elderly patient should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Close8.6 Renal Impairment
The dosing interval for tenofovir disoproxil fumarate should be modified in adult patients with estimated creatinine clearance below 50 mL/min or in patients with end stage renal disease requiring dialysis [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
-
10 OVERDOSAGEIf overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary. Tenofovir is efficiently removed by hemodialysis with an ...
If overdose occurs, the patient must be monitored for evidence of toxicity, and standard supportive treatment applied as necessary.
Close
Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. Following a single 300 mg dose of tenofovir disoproxil fumarate, a four-hour hemodialysis session removed approximately 10% of the administered tenofovir dose. -
11 DESCRIPTIONTenofovir disoproxil fumarate (TDF) (a prodrug of tenofovir) which is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. TDF is converted in vivo to tenofovir ...
Tenofovir disoproxil fumarate (TDF) (a prodrug of tenofovir) which is a fumaric acid salt of bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. TDF is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5’-monophosphate. Tenofovir exhibits activity against HIV-1 reverse transcriptase.
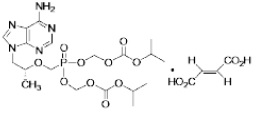
The chemical name of TDF is 9-[(R)-2-[[bis[[(isopropoxycarbonyl)oxy]methoxy]phosphinyl]methoxy]propyl]adenine fumarate (1:1). It has a molecular formula of C19H30N5O10P • C4H4O4 and a molecular weight of 635.52. It has the following structural formula:
Close
Tenofovir disoproxil fumarate is a white to off-white crystalline powder with a solubility of 13.4 mg/mL in distilled water at 25 °C. It has an octanol/phosphate buffer (pH 6.5) partition coefficient (log p) of 1.25 at 25 °C.
Tenofovir disoproxil fumarate is available as tablets.
Tenofovir disoproxil fumarate tablets are for oral administration and are available in strength of 300 mg of TDF, which are equivalent to 245 mg of tenofovir disoproxil.
Each tablet of tenofovir disoproxil fumarate contains the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The tablets are coated with Opadry II Blue 30K505001, which contains FD&C blue #2 aluminum lake, hypromellose, lactose monohydrate, titanium dioxide and triacetin.
In this insert, all dosages are expressed in terms of TDF except where otherwise noted. -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tenofovir disoproxil fumarate is an antiviral drug [see Microbiology (12.4)]. 12.3 Pharmacokinetics - The pharmacokinetics of TDF have been evaluated in healthy ...
12.1 Mechanism of Action
Tenofovir disoproxil fumarate is an antiviral drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetics of TDF have been evaluated in healthy volunteers and HIV-1 infected individuals. Tenofovir pharmacokinetics are similar between these populations.
Absorption
Tenofovir disoproxil fumarate is a water soluble diester prodrug of the active ingredient tenofovir. The oral bioavailability of tenofovir from tenofovir disoproxil fumarate in fasted subjects is approximately 25%. Following oral administration of a single dose of tenofovir disoproxil fumarate 300 mg to HIV-1 infected subjects in the fasted state, maximum serum concentrations (Cmax) are achieved in 1.0 ± 0.4 hrs. Cmax and AUC values are 0.30 ± 0.09 μg/mL and 2.29 ± 0.69 μg.hr/mL, respectively.The pharmacokinetics of tenofovir are dose proportional over a tenofovir disoproxil fumarate dose range of 75 to 600 mg and are not affected by repeated dosing.
In a single-dose bioequivalence study conducted under non-fasted conditions (dose administered with 4 oz. applesauce) in healthy adult volunteers, the mean Cmax of tenofovir was 26% lower for the oral powder relative to the tablet formulation. Mean AUC of tenofovir was similar between the oral powder and tablet formulations.
Distribution
In vitro binding of tenofovir to human plasma or serum proteins is less than 0.7 and 7.2%, respectively, over the tenofovir concentration range 0.01 to 25 μg/mL. The volume of distribution at steady-state is 1.3 ± 0.6 L/kg and 1.2 ± 0.4 L/kg, following intravenous administration of tenofovir 1.0 mg/kg and 3.0 mg/kg.
Metabolism and Elimination
In vitro studies indicate that neither tenofovir disoproxil nor tenofovir are substrates of CYP enzymes.
Following IV administration of tenofovir, approximately 70-80% of the dose is recovered in the urine as unchanged tenofovir within 72 hours of dosing. Following single dose, oral administration of tenofovir disoproxil fumarate, the terminal elimination half-life of tenofovir is approximately 17 hours. After multiple oral doses of tenofovir disoproxil fumarate 300 mg once daily (under fed conditions), 32 ± 10% of the administered dose is recovered in urine over 24 hours.
Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion. There may be competition for elimination with other compounds that are also renally eliminated.
Effects of Food on Oral Absorption
Administration of tenofovir disoproxil fumarate 300 mg tablets following a high-fat meal (~700 to 1,000 kcal containing 40 to 50% fat) increases the oral bioavailability, with an increase in tenofovir AUC0-∞ of approximately 40% and an increase in Cmax of approximately 14%. However, administration of tenofovir disoproxil fumarate with a light meal did not have a significant effect on the pharmacokinetics of tenofovir when compared to fasted administration of the drug. Food delays the time to tenofovir Cmax by approximately 1 hour. Cmax and AUC of tenofovir are 0.33 ± 0.12 μg/mL and 3.32 ± 1.37 μg•hr/mL following multiple doses of tenofovir disoproxil fumarate 300 mg once daily in the fed state, when meal content was not controlled.
Specific Populations
Race
There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations.Gender
Tenofovir pharmacokinetics are similar in male and female subjects.Pediatric Patients
2 Years and Older: Steady-state pharmacokinetics of tenofovir were evaluated in 31 HIV-1 infected pediatric subjects 2 years to less than 18 years of age (Table 13). Tenofovir exposure achieved in these pediatric subjects receiving oral once daily doses of tenofovir disoproxil fumarate 300 mg (tablet) was similar to exposures achieved in adults receiving once-daily doses of tenofovir disoproxil fumarate 300 mg.
Table 13 Mean (± SD) Tenofovir Pharmacokinetic Parameters by Age Groups for HIV-1-infected Pediatric Patients 2 years and older for the Tablet.Dose and Formulation
300 mg Tablet
12 Years to <18 Years (N=8)
Cmax (μg/mL)
0.38 ± 0.13
AUCtau (μg•hr/mL)
3.39 ± 1.22
Tenofovir exposures in HBV-infected pediatric subjects (12 years to less than 18 years of age) receiving oral once-daily doses of tenofovir disoproxil fumarate 300 mg tablet and pediatric subjects 2 years to less than 12 years of age receiving tenofovir disoproxil fumarate 8 mg/kg of body weight (tablet or powder) up to a maximum dose of 300 mg were comparable to exposures achieved in HIV-1 infected adult subjects receiving identical doses.
Geriatric Patients
Pharmacokinetic trials have not been performed in the elderly (65 years and older).
Patients with Renal Impairment
The pharmacokinetics of tenofovir are altered in subjects with renal impairment [see Warnings and Precautions (5.2)]. In subjects with creatinine
clearance below 50 mL/min or with end-stage renal disease (ESRD) requiring dialysis, Cmax , and AUC0-∞ of tenofovir were increased (Table 14).Table 14 Pharmacokinetic Parameters (Mean ± SD) of Tenofovira in Subjects with Varying Degrees of Renal Function
Baseline Creatinine Clearance (mL/min)
>80
N=3
50-80 N=10
30-49 N=8
12-29 N=11
Cmax (μg/mL)
0.34 ± 0.03
0.33 ± 0.06
0.37 ± 0.16
0.60 ± 0.19
AUC0-∞ (μg•hr/mL)
2.18 ± 0.26
3.06 ± 0.93
6.01 ± 2.50
15.98 ± 7.22
CL/F (mL/min)
1043.7 ± 115.4
807.7 ± 279.2
444.4 ± 209.8
177.0 ± 97.1
CLrenal (mL/min)
243.5 ± 33.3
168.6 ± 27.5
100.6 ± 27.5
43.0 ± 31.2
a. 300 mg, single dose of tenofovir disoproxil fumarate tablets
Patients with Hepatic Impairment
The pharmacokinetics of tenofovir following a 300 mg single dose of tenofovir disoproxil fumarate have been studied in non-HIV infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects. No change in tenofovir disoproxil fumarate dosing is required in patients with hepatic impairment.
Assessment of Drug Interactions
At concentrations substantially higher (~300-fold) than those observed in vivo, tenofovir did not inhibit in vitro drug metabolism mediated by any of the following human CYP isoforms: CYP3A4, CYP2D6, CYP2C9, or CYP2E1. However, a small (6%) but statistically significant reduction in metabolism of CYP1A substrate was observed. Based on the results of in vitro experiments and the known elimination pathway of tenofovir, the potential for CYP-mediated interactions involving tenofovir with other medicinal products is low.
Tenofovir disoproxil fumarate has been evaluated in healthy volunteers in combination with other antiretroviral and potential concomitant drugs. Tables 15 and 16 summarize pharmacokinetic effects of coadministered drug on tenofovir pharmacokinetics and effects of tenofovir disoproxil fumarate on the pharmacokinetics of coadministered drug.
TDF is a substrate of P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) transporters. When TDF is coadministered with an inhibitor of these transporters, an increase in absorption may be observed.
No clinically significant drug interactions have been observed between tenofovir disoproxil fumarate and efavirenz, methadone, nelfinavir, oral contraceptives, ribavirin, or sofosbuvir.
Table 15 Drug Interactions: Changes in Pharmacokinetic Parameters for Tenofovira in the Presence of the Coadministered DrugCoadministered Drug
Dose of Coadministered Drug (mg)
N
% Change of Tenofovir Pharmacokinetic Parametersb (90% CI)
Cmax
AUC
Cmin
Atazanavirc
400 once daily × 14 days
33
↑ 14 (↑ 8 to ↑ 20)
↑ 24 (↑ 21 to ↑ 28)
↑ 22 (↑ 15 to ↑ 30)
Atazanavir/ Ritonavirc
300/100 once daily
12
↑ 34 (↑ 20 to ↑ 51)
↑ 37 (↑ 30 to ↑ 45)
↑ 29 (↑ 21 to ↑ 36)
Darunavir/ Ritonavird
300/100 twice daily
12
↑ 24 (↑ 8 to ↑ 42)
↑ 22 (↑ 10 to ↑ 35)
↑ 37 (↑ 19 to ↑ 57)
Indinavir
800 three times daily × 7 days
13
↑ 14 (↓ 3 to ↑ 33)
↔
↔
Ledipasvir/ Sofosbuvire,f
90/400 once daily × 10 days
24
↑ 47 (↑ 37 to ↑ 58)
↑ 35 (↑ 29 to ↑ 42 )
↑ 47 (↑ 38 to ↑ 57)
Ledipasvir/ Sofosbuvire,g
23
↑ 64 (↑ 54 to ↑ 74)
↑ 50 (↑ 42 to ↑ 59)
↑ 59 (↑ 49 to ↑ 70)
Ledipasvir/ Sofosbuvirh
90/400 once daily × 14 days
15
↑ 79 (↑ 56 to ↑ 104)
↑ 98 (↑ 77 to ↑ 123)
↑ 163 (↑ 132 to↑197)
Lopinavir/ Ritonavir
400/100 twice daily × 14 days
24
↔
↑ 32 (↑ 25 to ↑ 38)
↑ 51 (↑ 37 to ↑ 66)
Saquinavir/ Ritonavir
1000/100 twice daily × 14 days
35
↔
↔
↑ 23 (↑ 16 to ↑ 30)
Sofosbuviri
400 single dose
16
↑ 25 (↑ 8 to ↑ 45)
↔
↔
Sofosbuvir/ Velpatasvirj
400/100 once daily
24
↑ 44 (↑ 33 to ↑ 55)
↑ 40 (↑ 34 to ↑ 46)
↑ 84 (↑ 76 to ↑ 92)
Sofosbuvir/ Velpatasvirk
400/100 once daily
30
↑ 46 (↑ 39 to ↑ 54)
↑ 40 (↑ 34 to ↑ 45)
↑ 70 (↑ 61 to ↑ 79)
Sofosbuvir/ Velpatasvir/ Voxilaprevirl
400/100/100 + Voxilaprevirm 100 once daily
29
↑ 48 (↑ 36 to ↑ 61)
↑ 39 (↑ 32 to↑ 46)
↑ 47 (↑ 38 to ↑ 56)
Tacrolimus
0.05 mg/kg twice daily × 7 days
21
↑ 13 (↑ 1 to ↑ 27)
↔
↔
Tipranavir/ Ritonavirn
500/100 twice daily
22
↓ 23 (↓ 32 to ↓ 13)
↓ 2 (↓ 9 to ↑ 5)
↑ 7 (↓ 2 to ↑ 17)
750/200 twice daily (23 doses)
20
↓ 38 (↓ 46 to ↓ 29)
↑ 2 (↓ 6 to ↑ 10)
↑ 14 (↑ 1 to ↑ 27)
a. Subjects received tenofovir disoproxil fumarate 300 mg once daily.
b. Increase = ↑; Decrease = ↓; No Effect = ⇔
c. Reyataz Prescribing Information.
d. Prezista Prescribing Information.
e. Data generated from simultaneous dosing with HARVONI (ledipasvir/sofosbuvir). Staggered administration (12 hours apart) provided similar results.
f. Comparison based on exposures when administered as atazanavir/ritonavir + FTC/TDF.
g. Comparison based on exposures when administered as darunavir/ritonavir + FTC/TDF.
h. Study conducted with ATRIPLA (EFV/FTC/TDF) coadministered with HARVONI; coadministration with HARVONI also results in comparable increases in tenofovir exposure when TDF is administered as COMPLERA (FTC /rilpivirine/TDF.), or TRUVADA + dolutegravir.
i. Study conducted with ATRIPLA coadministered with SOVALDI® (sofosbuvir).
j. Study conducted with COMPLERA coadministered with EPCLUSA; coadministration with EPCLUSA also results in comparable increases in tenofovir exposures when TDF is administered as ATRIPLA, STRIBILD, (elvitegravir/cobicistat/FTC/TDF), TRUVADA + atazanavir/ritonavir, or TRUVADA + darunavir/ritonavir.
k. Administered as raltegravir + FTC/TDF.
l. Comparison based on exposures when administered as darunavir + ritonavir + FTC/TDF.
m. Study conducted with additional voxilaprevir 100 mg to achieve voxilaprevir exposures expected in HCV-infected patients.
n. Aptivus Prescribing Information.
No effect on the pharmacokinetic parameters of the following coadministered drugs was observed with tenofovir disoproxil fumarate: abacavir, didanosine (buffered tablets), emtricitabine, entecavir and lamivudine.
Table 16 Drug Interactions: Changes in Pharmacokinetic Parameters for Coadministered Drug in the Presence of Tenofovir Disoproxil FumarateCoadministered Drug
Dose of Coadministered Drug (mg)
N
% Change of Coadministered Drug Pharmacokinetic Parametersa (90% CI)
Cmax
AUC
Cmin
Abacavir
300 once
8
↑ 12
(↓ 1 to ↑ 26)
ó
NA
Atazanavirb
400 once daily × 14 days
34
↓ 21
(↓ 27 to ↓ 14)
↓ 25
(↓ 30 to ↓ 19)
↓ 40
(↓ 48 to ↓ 32)
Atazanavirb
Atazanavir/ Ritonavir 300/100 once daily × 42 days
10
↓ 28
(↓ 50 to ↑ 5)
↓ 25c
(↓ 42 to ↓ 3)
↓ 23c
(↓ 46 to ↑ 10)
Darunavird
Darunavir/Ritonavir 300/100 once daily
12
↑ 16
(↓ 6 to ↑ 42)
↑ 21
↓ 5 to ↑ 54)
↑ 24
(↓ 10 to ↑ 69)
Didanosinee
250 once, simultaneously with tenofovir disoproxil fumarate and a light mealf
33
↓ 20g
(↓ 32 to ↓ 7)
óg
NA
Emtricitabine
200 once daily × 7 days
17
ó
ó
↑ 20
(↑ 12 to ↑ 29)
Entecavir
1 mg once daily x 10 days
28
ó
↑ 13
(↑ 11 to ↑ 15)
ó
Indinavir
800 three times daily × 7 days
12
↓ 11
(↓ 30 to ↑ 12)
ó
ó
Lamivudine
150 twice daily × 7 days
15
↓ 24
(↓ 34 to ↓ 12)
ó
ó
Lopinavir Ritonavir
Lopinavir/Ritonavir 400/100 twice daily × 14 days
24
ó
ó
ó
Saquinavir Ritonavir
Saquinavir/Ritonavir 1000/100 twice daily × 14 days
32
↑ 22
(↑ 6 to ↑ 41)
ó
↑ 29h
(↑ 12 to ↑ 48)
ó
↑ 47h
(↑ 23 to ↑ 76)
↑ 23
(↑ 3 to ↑ 46)
Tacrolimus
0.05 mg/kg twice daily x 7 days
21
ó
ó
ó
Tipranaviri
Tipranavir/Ritonavir 500/100 twice daily
22
↓ 17
(↓ 26 to ↓ 6)
↓ 18
(↓ 25 to ↓ 9)
↓ 21
(↓ 30 to ↓ 10)
Tipranavir/Ritonavir 750/200 twice daily (23 doses)
20
↓ 11
(↓ 16 to ↓ 4)
↓ 9
(↓ 15 to ↓ 3)
↓ 12
(↓ 22 to 0)
a. Increase = ↑; Decrease = ↓; No Effect =⇔ ; NA = Not Applicable
b. Reyataz Prescribing Information.
c. In HIV-infected subjects, addition of TDF to atazanavir 300 mg plus ritonavir 100 mg, resulted in AUC and Cmin values of atazanavir that were 2.3-and 4-fold higher than the respective values observed for atazanavir 400 mg when given alone.
d. Prezista Prescribing Information.
e. Videx EC Prescribing Information. Subjects received didanosine enteric-coated capsules.
f. 373 kcal, 8.2 g fat
g. Compared with didanosine (enteric-coated) 400 mg administered alone under fasting conditions.
h. Increases in AUC and Cmin are not expected to be clinically relevant; hence no dose adjustments are required when TDF and ritonavir-boosted saquinavir are coadministered.
i. Aptivus Prescribing Information.
Close12.4 Microbiology
Mechanism of Action
Tenofovir disoproxil fumarate is an acyclic nucleoside phosphonate diester analog of adenosine monophosphate. Tenofovir disoproxil fumarate requires initial diester hydrolysis for conversion to tenofovir and subsequent phosphorylations by cellular enzymes to form tenofovir diphosphate (TFV-DP), an obligate chain terminator. Tenofovir diphosphate inhibits the activity of HIV-1 reverse transcriptase (RT) and HBV RT by competing with the natural substrate deoxyadenosine 5’-triphosphate and, after incorporation into DNA, by DNA chain termination. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases α, β, and mitochondrial DNA polymerase γ.
Activity against HIV
Antiviral Activity
The antiviral activity of tenofovir against laboratory and clinical isolates of HIV-1 was assessed in lymphoblastoid cell lines, primary monocyte/macrophage cells and peripheral blood lymphocytes. The EC50 (50% effective concentration) values for tenofovir were in the range of 0.04 μM to 8.5 μM. In drug combination studies, tenofovir was not antagonistic with HIV-1 NRTIs (abacavir, didanosine, lamivudine, stavudine, zidovudine), NNRTIs (efavirenz, nevirapine), and protease inhibitors (amprenavir, indinavir, nelfinavir, ritonavir, saquinavir). Tenofovir displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, G, and O (EC50 values ranged from 0.5 μM to 2.2 μM) and strain-specific activity against HIV-2 (EC50 values ranged from 1.6 μM to 5.5 μM).
Resistance
HIV-1 isolates with reduced susceptibility to tenofovir have been selected in cell culture. These viruses expressed a K65R substitution in RT and showed a 2-to 4-fold reduction in susceptibility to tenofovir. In addition, a K70E substitution in HIV-1 RT has been selected by tenofovir and results in low-level reduced susceptibility to tenofovir.
In Trial 903 of treatment-naïve subjects (tenofovir disoproxil fumarate +3TC+EFV versus d4T+3TC+EFV) [see Clinical Studies (14.2)], genotypic analyses of isolates from subjects with virologic failure through Week 144 showed development of EFV and 3TC resistance-associated substitutions to occur most frequently and with no difference between the treatment arms. The K65R substitution occurred in 8/47 (17%) of analyzed patient isolates in the tenofovir disoproxil fumarate arm and in 2/49 (4%) of analyzed patient isolates in the d4T arm. Of the 8 subjects whose virus developed K65R in the tenofovir disoproxil fumarate arm through 144 weeks, 7 occurred in the first 48 weeks of treatment and one at Week 96. One patient in the tenofovir disoproxil fumarate arm developed the K70E substitution in the virus. Other substitutions resulting in resistance to tenofovir disoproxil fumarate were not identified in this trial.
In Trial 934 of treatment-naïve subjects (tenofovir disoproxil fumarate +FTC+EFV versus AZT/3TC+EFV) [see Clinical Studies (14.2)], genotypic analysis performed on HIV-1 isolates from all confirmed virologic failure subjects with >400 copies/mL of HIV-1 RNA at Week 144 or early discontinuation showed development of EFV resistance-associated substitutions occurred most frequently and was similar between the two treatment arms. The M184V substitution, associated with resistance to FTC and 3TC, was observed in 2/19 of analyzed subject isolates in the tenofovir disoproxil fumarate +FTC group and in 10/29 of analyzed subject isolates in the AZT/3TC group. Through 144 weeks of Trial 934, no subjects have developed a detectable K65R substitution in their HIV-1 as analyzed through standard genotypic analysis.
Cross Resistance
Cross resistance among certain HIV-1 NRTIs has been recognized. The K65R and K70E substitutions selected by tenofovir are also selected in some HIV-1 infected subjects treated with abacavir or didanosine. HIV-1 isolates with this substitution also show reduced susceptibility to FTC and 3TC. Therefore, cross resistance among these drugs may occur in patients whose virus harbors the K65R or K70E substitution. HIV-1 isolates from subjects (N=20) whose HIV-1 expressed a mean of three AZT-associated RT substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N), showed a 3.1-fold decrease in the susceptibility to tenofovir.
In Trials 902 and 907 conducted in treatment-experienced subjects (tenofovir disoproxil fumarate + Standard Background Therapy (SBT) compared to placebo + SBT) [see Clinical Studies (14.2)], 14/304 (5%) of the tenofovir disoproxil fumarate -treated subjects with virologic failure through Week 96 had >1.4-fold (median 2.7-fold) reduced susceptibility to tenofovir. Genotypic analysis of the baseline and failure isolates showed the development of the K65R substitution in the HIV-1 RT gene.
The virologic response to tenofovir disoproxil fumarate therapy has been evaluated with respect to baseline viral genotype (N=222) in treatment-experienced subjects participating in Trials 902 and 907. In these clinical trials, 94% of the participants evaluated had baseline HIV-1 isolates expressing at least one NRTI substitution. Virologic responses for subjects in the genotype substudy were similar to the overall trial results.
Several exploratory analyses were conducted to evaluate the effect of specific substitutions and substitutional patterns on virologic outcome. Because of the large number of potential comparisons, statistical testing was not conducted. Varying degrees of cross resistance of tenofovir disoproxil fumarate to pre-existing AZT resistance-associated substitutions (M41L, D67N, K70R, L210W, T215Y/F, or K219Q/E/N) were observed and appeared to depend on the type and number of specific substitutions. tenofovir disoproxil fumarate -treated subjects whose HIV-1 expressed 3 or more AZT resistance-associated substitutions that included either the M41L or L210W RT substitution showed reduced responses to tenofovir disoproxil fumarate therapy; however, these responses were still improved compared with placebo. The presence of the D67N, K70R, T215Y/F, or K219Q/E/N substitution did not appear to affect responses to tenofovir disoproxil fumarate therapy. Subjects whose virus expressed an L74V substitution without AZT resistance-associated substitutions (N=8) had reduced response to tenofovir disoproxil fumarate. Limited data are available for subjects whose virus expressed a Y115F substitution (N=3), Q151M substitution (N=2), or T69 insertion (N=4), all of whom had a reduced response.
In the protocol defined analyses, virologic response to tenofovir disoproxil fumarate was not reduced in subjects with HIV-1 that expressed the abacavir/FTC/3TC resistance-associated M184V substitution. HIV-1 RNA responses among these subjects were durable through Week 48.
Trials 902 and 907 Phenotypic Analyses
Phenotypic analysis of baseline HIV-1 from treatment-experienced subjects (N=100) demonstrated a correlation between baseline susceptibility to tenofovir disoproxil fumarate and response to tenofovir disoproxil fumarate therapy. Table 17 summarizes the HIV-1 RNA response by baseline tenofovir disoproxil fumarate susceptibility.
Table 17 HIV-1 RNA Response at Week 24 by Baseline Tenofovir Disoproxil Fumarate Susceptibility (Intent-To-Treat)aBaseline Tenofovir Disoproxil Fumarate Susceptibilityb
Change in HIV-1 RNAc (N)
<1
>1 and ≤3
>3 and ≤4
>4
-0.74 (35)
-0.56 (49)
-0.3 (7)
-0.12 (9)
a. Tenofovir susceptibility was determined by recombinant phenotypic Antivirogram assay (Virco).
b. Fold change in susceptibility from wild-type.
c. Average HIV-1 RNA change from baseline through Week 24 (DAVG24) in log10 copies/mL.Activity against HBV
Antiviral Activity
The antiviral activity of tenofovir against HBV was assessed in the HepG2 2.2.15 cell line. The EC50 values for tenofovir ranged
from 0.14 to 1.5 μM, with CC50 (50% cytotoxicity concentration) values >100 μM. In cell culture combination antiviral activity studies of tenofovir with HBV NRTIs entecavir, lamivudine, and telbivudine, and with the HIV-1 NRTIs emtricitabine, no antagonistic activity was observed.Resistance
Cumulative tenofovir disoproxil fumarate genotypic resistance has been evaluated annually for up to 384 weeks in Trials 0102, 0103, 0106, 0108, and 0121 [see Clinical Studies (14.4)] with the paired HBV rt amino acid sequences of the pretreatment and on-treatment isolates from subjects who received at least 24 weeks of tenofovir disoproxil fumarate monotherapy and remained viremic with HBV DNA ≥400 copies/mL (69 IU/mL) at the end of each study year (or at discontinuation of tenofovir disoproxil fumarate monotherapy) using an as-treated analysis.
In the nucleotide-naïve population from Trials 0102 and 0103, HBeAg-positive subjects had a higher baseline viral load than HBeAg-negative subjects and a significantly higher proportion of the subjects remained viremic at their last time point on tenofovir disoproxil fumarate monotherapy (15% versus 5%, respectively).HBV isolates from these subjects who remained viremic showed treatment-emergent substitutions (Table 18); however, no specific substitutions occurred at a sufficient frequency to be associated with resistance to tenofovir disoproxil fumarate (genotypic and phenotypic analyses).
Table 18 Amino Acid Substitutions in Viremic Subjects across HBV Trials of Tenofovir Disoproxil Fumarate.
Compensated Liver Disease
Decompensated Liver Disease (N=39)d
Nucleotide-Naïve (N=417)a
HEPSERA-Experienced (N=247)b
Lamivudine-Resistant (N=136)c
Viremic at Last Time
Point on Tenofovir Disoproxil Fumarate
38/417 (9%)
37/247 (15%)
9/136 (7%)
7/39 (18%)
Treatment-Emergent Amino Acid Substitutionse
18f/32 (56%)
11g/31 (35%)
6h/8 (75%)
3/5 (60%)
a. Nucleotide-naïve subjects from Trials 0102 (N=246) and 0103 (N=171) receiving up to 384 weeks of treatment with tenofovir disoproxil fumarate.
b. HEPSERA-experienced subjects from Trials 0102/0103 (N=195) and 0106 (N=52) receiving up to 336 weeks of treatment with tenofovir disoproxil fumarate after switching to tenofovir disoproxil fumarate from HEPSERA. Trial 0106, a randomized, double-blind, 168-week Phase 2 trial, has been completed.
c. Lamivudine-resistant subjects from Trial 0121 (N=136) receiving up to 96 weeks of treatment with tenofovir disoproxil fumarate after switching to tenofovir disoproxil fumarate from lamivudine.
d. Subjects with decompensated liver disease from Trial 0108 (N=39) receiving up to 48 weeks of treatment with tenofovir disoproxil fumarate.
e. Denominator includes those subjects who were viremic at last time point on tenofovir disoproxil fumarate monotherapy and had evaluable paired genotypic data.
f. Of the 18 subjects with treatment-emergent amino acid substitutions during Trials 0102 and 0103, 5 subjects had substitutions at conserved sites and 13 subjects had substitutions only at polymorphic sites, and 8 subjects had only transient substitutions that were not detected at the last time point on tenofovir disoproxil fumarate.
g. Of the 11 HEPSERA-experienced subjects with treatment-emergent amino acid substitutions, 2 subjects had substitutions at conserved sites and 9 had substitutions only at polymorphic sites.
h. Of the 6 lamivudine-resistant subjects with treatment-emergent substitutions during Trial 0121, 3 subjects had substitutions at conserved sites and 3 had substitutions only at polymorphic sites.
Cross Resistance
Cross resistance has been observed between HBV NRTIs.
In cell-based assays, HBV strains expressing the rtV173L, rtL180M, and rtM204I/V substitutions associated with resistance to lamivudine (3TC) and telbivudine showed a susceptibility to tenofovir ranging from 0.7-to 3.4-fold that of wild type virus. The rtL180M and rtM204I/V double substitutions conferred 3.4-fold reduced susceptibility to tenofovir.
HBV strains expressing the rtL180M, rtT184G, rtS202G/I, rtM204V, and rtM250V substitutions associated with resistance to entecavir showed a susceptibility to tenofovir ranging from 0.6-to 6.9-fold that of wild type virus.
HBV strains expressing the adefovir resistance-associated substitutions rtA181V and/or rtN236T showed reductions in susceptibility to tenofovir ranging from 2.9-to 10-fold that of wild type virus. Strains containing the rtA181T substitution showed changes in susceptibility to tenofovir ranging from 0.9-to 1.5-fold that of wild type virus.
One hundred fifty-two subjects initiating tenofovir disoproxil fumarate therapy in Trials 0102, 0103, 0106, 0108, and 0121 harbored HBV with known resistance substitutions to HBV NRTIs: 14 with adefovir resistance-associated substitutions (rtA181S/T/V and/or rtN236T), 135 with 3TC resistance-associated substitutions (rtM204I/V), and 3 with both adefovir and 3TC resistance-associated substitutions. Following up to 384 weeks of tenofovir disoproxil fumarate treatment, 10 of the 14 subjects with adefovir-resistant HBV, 124 of the 135 subjects with 3TC-resistant HBV, and 2 of the 3 subjects with both adefovir-and 3TC-resistant HBV achieved and maintained virologic suppression (HBV DNA <400 copies/mL [69 IU/mL]). Three of the 5 subjects whose virus harbored both the rtA181T/V and rtN236T substitutions remained viremic. -
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility - Carcinogenesis - Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 16 ...
13.1 Carcinogenesis & Mutagenesis & Impairment of Fertility
Carcinogenesis
Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 16 times (mice) and 5 times (rats) those observed in humans at the therapeutic dose for HIV-1 infection. At the high dose in female mice, liver adenomas were increased at exposures 16 times that in humans. In rats, the study was negative for carcinogenic findings at exposures up to 5 times that observed in humans at the therapeutic dose.
Mutagenesis
Tenofovir disoproxil fumarate was mutagenic in the in vitro mouse lymphoma assay and negative in an in vitro bacterial mutagenicity test (Ames test). In an in vivo mouse micronucleus assay, TDF was negative when administered to male mice.
Impairment of Fertility
There were no effects on fertility, mating performance or early embryonic development when TDF was administered to male rats at a dose equivalent to 10 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 15 days prior to mating through day seven of gestation. There was, however, an alteration of the estrous cycle in female rats.Close13.2 Animal Toxicology and/or Pharmacology
Tenofovir and TDF administered in toxicology studies to rats, dogs, and monkeys at exposures (based on AUCs) greater than or equal to 6 fold those observed in humans caused bone toxicity. In monkeys the bone toxicity was diagnosed as osteomalacia. Osteomalacia observed in monkeys appeared to be reversible upon dose reduction or discontinuation of tenofovir. In rats and dogs, the bone toxicity manifested as reduced bone mineral density. The mechanism(s) underlying bone toxicity is unknown.
Evidence of renal toxicity was noted in 4 animal species. Increases in serum creatinine, BUN, glycosuria, proteinuria, phosphaturia, and/or calciuria and decreases in serum phosphate were observed to varying degrees in these animals. These toxicities were noted at exposures (based on AUCs) 2-20 times higher than those observed in humans. The relationship of the renal abnormalities, particularly the phosphaturia, to the bone toxicity is not known. -
14 CLINICAL STUDIES14.1 Overview of Clinical Trials - The efficacy and safety of tenofovir disoproxil fumarate in adults and pediatric subjects were evaluated in the trials summarized in Table 19. Table 19 Trials ...
14.1 Overview of Clinical Trials
The efficacy and safety of tenofovir disoproxil fumarate in adults and pediatric subjects were evaluated in the trials summarized in Table 19.
Table 19 Trials Conducted with Tenofovir Disoproxil Fumarate in Adults and Pediatric Subjects for HIV-1 Treatment and Chronic HBV TreatmentTrial
Population
Study Arms (N)a
Timepoint (Week)
Trial 903b (NCT00158821)
HIV-1 treatment-naïve adults
Tenofovir Disoproxil Fumarate +lamivudine+efavirenz (299) stavudine+lamivudine+efavirenz (301)
144
Trial 934c (NCT00112047)
emtricitabine+ Tenofovir Disoproxil Fumarate +efavirenz (257) zidovudine/lamivudine+efavirenz (254)
144
Trial 907d (NCT00002450)
HIV-1 treatment-experienced adults
Tenofovir Disoproxil Fumarate (368) Placebo (182)
24
Trial 0102b (NCT00117676)
HBeAg-negative adults with chronic HBV
Tenofovir Disoproxil Fumarate (250) HEPSERA (125)
48
Trial 0103b (NCT00116805)
HBeAg-positive adults with chronic HBV
Tenofovir Disoproxil Fumarate (176) HEPSERA (90)
48
Trial 121b (NCT00737568)
Adults with lamivudine-resistant chronic HBV
Tenofovir Disoproxil Fumarate (141)
96
Trial 0108b (NCT00298363)
Adults with chronic HBV and decompensated liver disease
Tenofovir Disoproxil Fumarate (45)
48
Trial 352c (NCT00528957)
HIV-1 treatment experienced pediatric subjects 2 years to <12 years
Tenofovir Disoproxil Fumarate (44) stavudine or zidovudine (48)
48
Trial 321d (NCT00352053)
HIV-1 treatment-experienced pediatric subjects 12 years to <18 years
Tenofovir Disoproxil Fumarate (45) Placebo (42)
48
Trial 115d (NCT00734162)
Pediatric subjects 12 years to <18 years with chronic HBV
Tenofovir Disoproxil Fumarate (52) Placebo (54)
72
Trial 144d
(NCT01651403)
Pediatric subjects
2 years to <12
years with
chronic HBV
Tenofovir
Disoproxil
Fumarate (60)
Placebo (29)
48a. Randomized and dosed.
b. Randomized, double-blind, active-controlled trial.
c. Randomized, open-label active-controlled trial.
d. Randomized, double-blind, placebo-controlled trial.14.2 Clinical Trial Results in Adults with HIV-1 Infection
Treatment-Naïve Subjects: Trial 903
Data through 144 weeks are reported for Trial 903, a double-blind, active-controlled multicenter trial comparing tenofovir disoproxil fumarate (300 mg once daily) administered in combination with lamivudine (3TC) and efavirenz (EFV) versus stavudine (d4T), 3TC, and EFV in 600 antiretroviral-naïve subjects. Subjects had a mean age of 36 years (range 18−64); 74% were male, 64% were Caucasian, and 20% were Black. The mean baseline CD4+ cell count was 279 cells/mm3 (range 3−956) and median baseline plasma HIV-1 RNA was 77,600 copies/mL (range 417−5,130,000). Subjects were stratified by baseline HIV-1 RNA and CD4+ cell count. Forty-three percent of subjects had baseline viral loads >100,000 copies/mL and 39% had CD4+ cell counts <200 cells/mm3. Table 20 provides treatment outcomes through 48 and 144 weeks.
Table 20 Outcomes of Randomized Treatment at Week 48 and 144 (Trial 903)Outcomes
At Week 48
At Week 144
Tenofovir Disoproxil Fumarate +
3TC +EFV (N=299)
d4T+3TC +EFV (N=301)
Tenofovir Disoproxil Fumarate +3TC +EFV (N=299)
d4T+3TC +EFV (N=301)
Respondera
79%
82%
68%
62%
Virologic failureb
6%
4%
10%
8%
Rebound
5%
3%
8%
7%
Never suppressed
0%
1%
0%
0%
Added an antiretroviral agent
1%
1%
2%
1%
Death
<1%
1%
<1%
2%
Discontinued due to adverse event
6%
6%
8%
13%
Discontinued for other reasonsc
8%
7%
14%
15%
a. Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Week 48 and 144.
b. Includes confirmed viral rebound and failure to achieve confirmed <400 copies/mL through Week 48 and 144.
c. Includes lost to follow-up, subject’s withdrawal, noncompliance, protocol violation and other reasons.
Achievement of plasma HIV-1 RNA concentrations of <400 copies/mL at Week 144 was similar between the two treatment groups for the population stratified at baseline on the basis of HIV-1 RNA concentration (> or ≤100,000 copies/mL) and CD4+ cell count (< or ≥200 cells/mm3). Through 144 weeks of therapy, 62% and 58% of subjects in the tenofovir disoproxil fumarate and d4T arms, respectively, achieved and maintained confirmed HIV-1 RNA <50 copies/mL. The mean increase from baseline in CD4+ cell count was 263 cells/mm3 for the tenofovir disoproxil fumarate arm and 283 cells/mm3 for the d4T arm.
Through 144 weeks, 11 subjects in the tenofovir disoproxil fumarate group and 9 subjects in the d4T group experienced a new CDC Class C event.
Treatment-Naïve Subjects: Trial 934
Data through 144 weeks are reported for Trial 934, a randomized, open-label, active-controlled multicenter trial comparing emtricitabine (FTC) + tenofovir disoproxil fumarate administered in combination with efavirenz (EFV) versus zidovudine (AZT)/lamivudine (3TC) fixed-dose combination administered in combination with EFV in 511 antiretroviral-naïve subjects. From Weeks 96 to 144 of the trial, subjects received a fixed-dose combination of FTC and TDF with EFV in place of FTC + tenofovir disoproxil fumarate with EFV. Subjects had a mean age of 38 years (range 18−80); 86% were male, 59% were Caucasian, and 23% were Black. The mean baseline CD4+ cell count was 245 cells/mm3 (range 2−1191) and median baseline plasma HIV-1 RNA was 5.01 log10 copies/mL (range 3.56−6.54). Subjects were stratified by baseline CD4+ cell count (< or ≥200 cells/mm3); 41% had CD4+ cell counts <200 cells/mm3 and 51% of subjects had baseline viral loads >100,000 copies/mL. Table 21 provides treatment outcomes through 48 and 144 weeks for those subjects who did not have EFV resistance at baseline.
Table 21 Outcomes of Randomized Treatment at Week 48 and 144 (Trial 934)Outcomes
At Week 48
At Week 144
FTC +Tenofovir Disoproxil Fumarate +EFV (N=244)
AZT/3TC +EFV (N=243)
FTC + Tenofovir Disoproxil Fumarate +EFV (N=227)a
AZT/3TC +EFV (N=229)a
Responderb
84%
73%
71%
58%
Virologic failurec
2%
4%
3%
6%
Rebound
1%
3%
2%
5%
Never suppressed
0%
0%
0%
0%
Change in antiretroviral regimen
1%
1%
1%
1%
Death
<1%
1%
1%
1%
Discontinued due to adverse event
4%
9%
5%
12%
Discontinued for other reasonsd
10%
14%
20%
22%
a. Subjects who were responders at Week 48 or Week 96 (HIV-1 RNA <400 copies/mL) but did not consent to continue the trial after Week 48 or Week 96 were excluded from analysis.
b. Subjects achieved and maintained confirmed HIV-1 RNA <400 copies/mL through Weeks 48 and 144.
c. Includes confirmed viral rebound and failure to achieve confirmed <400 copies/mL through Weeks 48 and 144.
d. Includes lost to follow-up, subject withdrawal, noncompliance, protocol violation and other reasons.Through Week 48, 84% and 73% of subjects in the FTC + tenofovir disoproxil fumarate group and the AZT/3TC group, respectively, achieved and maintained HIV-1 RNA <400 copies/mL (71% and 58% through Week 144). The difference in the proportion of subjects who achieved and maintained HIV-1 RNA <400 copies/mL through 48 weeks largely results from the higher number of discontinuations due to adverse events and other reasons in the AZT/3TC group in this open-label trial. In addition, 80% and 70% of subjects in the FTC + tenofovir disoproxil fumarate group and the AZT/3TC group, respectively, achieved and maintained HIV-1 RNA <50 copies/mL through Week 48 (64% and 56% through Week 144). The mean increase from baseline in CD4+ cell count was 190 cells/mm3 in the FTC + tenofovir disoproxil fumarate group and 158 cells/mm3 in the AZT/3TC group at Week 48 (312 and 271 cells/mm3 at Week 144).
Through 48 weeks, 7 subjects in the FTC + tenofovir disoproxil fumarate group and 5 subjects in the AZT/3TC group experienced a new CDC Class C event (10 and 6 subjects through 144 weeks).Treatment-Experienced Subjects: Trial 907
Trial 907 was a 24-week, double-blind, placebo-controlled multicenter trial of tenofovir disoproxil fumarate added to a stable background regimen of antiretroviral agents in 550 treatment-experienced subjects. After 24 weeks of blinded trial treatment, all subjects continuing on trial were offered open-label tenofovir disoproxil fumarate for an additional 24 weeks. Subjects had a mean baseline CD4+ cell count of 427 cells/mm3 (range 23−1,385), median baseline plasma HIV-1 RNA of 2,340 (range 50−75,000) copies/mL, and mean duration of prior HIV-1 treatment was 5.4 years. Mean age of the subjects was 42 years; 85% were male, 69% Caucasian, 17% Black, and 12% Hispanic.Table 22 provides the percent of subjects with HIV-1 RNA <400 copies/mL and outcomes of subjects through 48 weeks.
Table 22 Outcomes of Randomized Treatment (Trial 907)
Outcomes
0-24 weeks
0-48 weeks
24-48 weeks
Tenofovir Disoproxil Fumarate (N=368)
Placebo (N=182)
Tenofovir Disoproxil Fumarate (N=368)
Placebo Crossover to Tenofovir Disoproxil Fumarate (N=170)
HIV-1 RNA <400 copies/mL a
40%
11%
28%
30%
Virologic failureb
53%
84%
61%
64%
Discontinued due to adverse event
3%
3%
5%
5%
Discontinued for other reasonsc
3%
3%
5%
1%a. Subjects with HIV-1 RNA <400 copies/mL and no prior study drug discontinuation at Week 24 and 48, respectively.
b. Subjects with HIV-1 RNA ≥400 copies/mL efficacy failure or missing HIV-1 RNA at Week 24 and 48, respectively.
c. Includes lost to follow-up, subject withdrawal, noncompliance, protocol violation, and other reasons.
At 24 weeks of therapy, there was a higher proportion of subjects in the tenofovir disoproxil fumarate arm compared to the placebo arm with HIV-1 RNA <50 copies/mL (19% and 1%, respectively). Mean change in absolute CD4+ cell counts by Week 24 was +11 cells/mm3 for the tenofovir disoproxil fumarate group and −5 cells/mm3 for the placebo group. Mean change in absolute CD4+ cell counts by Week 48 was +4 cells/mm3 for the tenofovir disoproxil fumarate group.
Through Week 24, one subject in the tenofovir disoproxil fumarate group and no subjects in the placebo group experienced a new CDC Class C event.
14.3 Clinical Trial Results in Pediatric Subjects with HIV-1 Infection
In Trial 352, 92 treatment-experienced subjects 2 years to less than 12 years of age with stable, virologic suppression on a stavudine (d4T)-or zidovudine (AZT)-containing regimen were randomized to either replace d4T or AZT with tenofovir disoproxil fumarate (N=44) or continue their original regimen (N=48) for 48 weeks. Five additional subjects over the age of 12 years were enrolled and randomized (tenofovir disoproxil fumarate N=4, original regimen N=1) but are not included in the efficacy analysis. After 48 weeks, all eligible subjects were allowed to continue in the trial receiving open-label tenofovir disoproxil fumarate. At Week 48, 89% of subjects in the tenofovir disoproxil fumarate treatment group and 90% of subjects in the d4T or AZT treatment group had HIV-1 RNA concentrations <400 copies/mL. During the 48-week randomized phase of the trial, 1 subject in the tenofovir disoproxil fumarate group discontinued the trial prematurely because of virologic failure/lack of efficacy and 3 subjects (2 subjects in the tenofovir disoproxil fumarate group and 1 subject in the d4T or AZT group) discontinued for other reasons.
In Trial 321, 87 treatment-experienced subjects 12 years to less than 18 years of age were treated with tenofovir disoproxil fumarate (N=45) or placebo (N=42) in combination with an optimized background regimen (OBR) for 48 weeks. The mean baseline CD4 cell count was 374 cells/mm3 and the mean baseline plasma HIV-1 RNA was 4.6 log10 copies/mL. At baseline, 90% of subjects harbored NRTI resistance-associated substitutions in their HIV-1 isolates. Overall, the trial failed to show a difference in virologic response between the tenofovir disoproxil fumarate and placebo groups. Subgroup analyses suggest the lack of difference in virologic response may be attributable to imbalances between treatment arms in baseline viral susceptibility to tenofovir disoproxil fumarate and OBR.
Although changes in HIV-1 RNA in these highly treatment-experienced subjects were less than anticipated, the comparability of the pharmacokinetic and safety data to that observed in adults supports the use of tenofovir disoproxil fumarate in pediatric patients 12 years and older who weigh at least 35 kg and whose HIV-1 isolate is expected to be sensitive to tenofovir disoproxil fumarate [see Warnings and Precautions (5.5), Adverse Reactions (6.1), and Clinical Pharmacology (12.3)].14.4 Clinical Trial Results in Adults with Chronic Hepatitis B
HBeAg-Negative Chronic HBV Subjects: Trial 0102
Trial 0102 was a Phase 3, randomized, double-blind, active-controlled trial of tenofovir disoproxil fumarate 300 mg compared to HEPSERA 10 mg in 375 HBeAg-(anti-HBe+) subjects with compensated liver function, the majority of whom were nucleoside-naïve. The mean age of subjects was 44 years; 77% were male, 25% were Asian, 65% were Caucasian, 17% had previously received alpha-interferon therapy, and 18% were nucleoside-experienced (16% had prior lamivudine experience). At baseline, subjects had a mean Knodell necroinflammatory score of 7.8; mean plasma HBV DNA was 6.9 log10 copies/mL; and mean serum ALT was 140 U/L.
HBeAg-Positive Chronic HBV Subjects: Trial 0103
Trial 0103 was a Phase 3, randomized, double-blind, active-controlled trial of tenofovir disoproxil fumarate 300 mg compared to HEPSERA 10 mg in 266 HBeAg+ nucleoside-naïve subjects with compensated liver function. The mean age of subjects was 34 years; 69% were male, 36% were Asian, 52% were Caucasian, 16% had previously received alpha-interferon therapy, and <5% were nucleoside experienced. At baseline, subjects had a mean Knodell necroinflammatory score of 8.4; mean plasma HBV DNA was 8.7 log10 copies /mL; and mean serum ALT was 147 U/L.
The primary data analysis was conducted after all subjects reached 48 weeks of treatment and results are summarized below.
The primary efficacy endpoint in both trials was complete response to treatment defined as HBV DNA <400 copies/mL (69 IU/mL) and Knodell necroinflammatory score improvement of at least 2 points, without worsening in Knodell fibrosis at Week 48 (see Table 23).
Table 23 Histological, Virological, Biochemical, and Serological Response at Week 48 (Trials 0102 and 0103)
0102 (HBeAg-)
0103 (HBeAg+)
Tenofovir Disoproxil Fumarate (N=250)
HEPSERA (N=125)
Tenofovir Disoproxil Fumarate (N=176)
HEPSERA (N=90)
Complete Response
71%
49%
67%
12%
Histology Histological Responsea
72%
69%
74%
68%
HBV DNA <400 copies/mL (<69 IU/mL)
93%
63%
76%
13%
ALT Normalized ALTb
76%
77%
68%
54%
Serology HBeAg Loss/ Seroconversion
NAc
NAc
20%/19%
16%/16%
HBsAg Loss/ Seroconversion
0/0
0/0
3%/1%
0/0
a. Knodell necroinflammatory score improvement of at least 2 points without worsening in Knodell fibrosis.
b. The population used for analysis of ALT normalization included only subjects with ALT above ULN at baseline.
c. NA = Not Applicable
Treatment Beyond 48 Weeks: Trials 0102 and 0103
In Trials 0102 (HBeAg-negative) and 0103 (HBeAg-positive), subjects who completed double-blind treatment (389 and 196 subjects who were originally randomized to tenofovir disoproxil fumarate and HEPSERA, respectively) were eligible to roll over to open-label tenofovir disoproxil fumarate with no interruption in treatment.
In Trial 0102, 266 of 347 subjects who entered the open-label period (77%) continued in the trial through Week 384. Among subjects randomized to tenofovir disoproxil fumarate followed by open-label treatment with tenofovir disoproxil fumarate, 73% had HBV DNA <400 copies/ml (69 IU/ml), and 63% had ALT normalization at Week 384. Among subjects randomized to HEPSERA followed by open-label treatment with tenofovir disoproxil fumarate, 80% had HBV DNA <400 copies/mL (69 IU/mL) and 70% had ALT normalization through Week 384. At Week 384, both HBsAg loss and seroconversion were approximately 1% in both treatment groups.
In Trial 0103, 146 of 238 subjects who entered the open-label period (61%) continued in the trial through Week 384. Among subjects randomized to tenofovir disoproxil fumarate, 49% had HBV DNA <400 copies/mL (69 IU/mL), 42% had ALT normalization, and 20% had HBeAg loss (13% seroconversion to anti-HBe antibody) through Week 384. Among subjects randomized to HEPSERA followed by open-label treatment with tenofovir disoproxil fumarate, 56% had HBV DNA <400 copies/mL (69 IU/mL), 50% had ALT normalization, and 28% had HBeAg loss (19% seroconversion to anti-HBe antibody) through Week 384. At Week 384, HBsAg loss and seroconversion were 11% and 8%, respectively, in subjects initially randomized to tenofovir disoproxil fumarate and 12% and 10%, respectively, in subjects initially randomized to HEPSERA.
Of the originally randomized and treated 641 subjects in the two trials, liver biopsy data from 328 subjects who received continuing open-label treatment with tenofovir disoproxil fumarate monotherapy were available for analysis at baseline, Week 48, and Week 240. There were no apparent differences between the subset of subjects who had liver biopsy data at Week 240 and those subjects remaining on open-label tenofovir disoproxil fumarate without biopsy data that would be expected to affect histological outcomes at Week 240. Among the 328 subjects evaluated, the observed histological response rates were 80% and 88% at Week 48 and Week 240, respectively. In the subjects without cirrhosis at baseline (Ishak fibrosis score 0-4), 92% (216/235) and 95% (223/235) had either improvement or no change in Ishak fibrosis score at Week 48 and Week 240, respectively. In subjects with cirrhosis at baseline (Ishak fibrosis score 5-6), 97% (90/93) and 99% (92/93) had either improvement or no change in Ishak fibrosis score at Week 48 and Week 240, respectively. Twenty-nine percent (27/93) and 72% (67/93) of subjects with cirrhosis at baseline experienced regression of cirrhosis by Week 48 and Week 240, respectively, with a reduction in Ishak fibrosis score of at least 2 points. No definitive conclusions can be established about the remaining study population who were not part of this subset analysis.
Lamivudine-Resistant Chronic HBV Subjects: Trial 121
Trial 121 was a randomized, double-blind, active-controlled trial evaluating the safety and efficacy of tenofovir disoproxil fumarate compared to an unapproved antiviral regimen in subjects with chronic hepatitis B, persistent viremia (HBV DNA ≥1,000 IU/mL), and genotypic evidence of lamivudine resistance (rtM204I/V +/-rtL180M). One hundred forty-one adult subjects were randomized to the tenofovir disoproxil fumarate treatment arm. The mean age of subjects randomized to tenofovir disoproxil fumarate was 47 years (range 18-73); 74% were male, 59% were Caucasian, and 37% were Asian. At baseline, 54% of subjects were HBeAg-negative, 46% were HBeAg-positive, and 56% had abnormal ALT. Subjects had a mean HBV DNA of 6.4 log10 copies/mL and mean serum ALT of 71 U/L at baseline.
After 96 weeks of treatment, 126 of 141 subjects (89%) randomized to tenofovir disoproxil fumarate had HBV DNA <400 copies/mL (69 IU/mL), and 49 of 79 subjects (62%) with abnormal ALT at baseline had ALT normalization. Among the HBeAg-positive subjects randomized to tenofovir disoproxil fumarate, 10 of 65 subjects (15%) experienced HBeAg loss and 7 of 65 subjects (11%) experienced anti-HBe seroconversion through Week 96. The proportion of subjects with HBV DNA concentrations below 400 copies/mL (69 IU/mL) at Week 96 was similar between the tenofovir disoproxil fumarate monotherapy and the comparator arms.Across the combined chronic hepatitis B treatment trials, the number of subjects with adefovir-resistance associated substitutions at baseline was too small to establish efficacy in this subgroup.
Chronic HBV and Decompensated Liver Disease Subjects: Trial 0108
Trial 0108 was a small randomized, double-blind, active-controlled trial evaluating the safety of tenofovir disoproxil fumarate compared to other antiviral drugs in subjects with chronic hepatitis B and decompensated liver disease through 48 weeks.Forty-five adult subjects (37 males and 8 females) were randomized to the tenofovir disoproxil fumarate treatment arm. At baseline, 69% of subjects were HBeAg-negative and 31% were HBeAg-positive. Subjects had a mean Child-Pugh score of 7, a mean MELD score of 12, mean HBV DNA of 5.8 log10 copies/mL, and mean serum ALT of 61 U/L at baseline. Trial endpoints were discontinuation due to an adverse event and confirmed increase in serum creatinine ≥0.5 mg/dL or confirmed serum phosphorus of <2 mg/dL [see Adverse Reactions (6.1)].
At 48 weeks, 31/44 (70%) and 12/26 (46%) tenofovir disoproxil fumarate -treated subjects achieved an HBV DNA <400 copies/mL (69 IU/mL), and normalized ALT, respectively. The trial was not designed to evaluate treatment impact on clinical endpoints such as progression of liver disease, need for liver transplantation, or death.
Close14.5 Clinical Trial Results in Pediatric Subjects with Chronic Hepatitis B
Pediatric Subjects 12 Years to less than 18 Years of Age with Chronic HBV
In Trial 115, 106 HBeAg negative (9%) and positive (91%) subjects aged 12 to less than 18 years with chronic HBV infection were randomized to receive blinded treatment with tenofovir disoproxil fumarate 300 mg (N=52) or placebo (N=54) for 72 weeks. At trial entry, the mean HBV DNA was 8.1 log10 copies/mL and mean ALT was 101 U/L. Of 52 subjects treated with tenofovir disoproxil fumarate, 20 subjects were nucleos(t)ide-naïve and 32 subjects were nucleos(t)ide-experienced. Thirty-one of the 32 nucleos(t)ide-experienced subjects had prior lamivudine experience. At Week 72, 88% (46/52) of subjects in the tenofovir disoproxil fumarate group and 0% (0/54) of subjects in the placebo group had HBV DNA <400 copies/mL (69 IU/mL). Among subjects with abnormal ALT at baseline, 74% (26/35) of subjects receiving tenofovir disoproxil fumarate had normalized ALT at Week 72 compared to 31% (13/42) in the placebo group. One tenofovir disoproxil fumarate -treated subject experienced sustained HBsAg-loss and seroconversion to anti-HBs during the first 72 weeks of trial participation.Pediatric Subjects 2 Years to less than 12 Years of Age with Chronic HBV
In Trial 144, 89 HBeAg positive (96%) and negative (4%) subjects 2 years to less than 12 years of age with chronic HBV infection were treated with tenofovir disoproxil fumarate 8 mg/kg up to a maximum dose of 300 mg (N=60) or placebo (N=29) once daily for 48 weeks. At trial entry, the mean HBV DNA was 8.1 log10 IU/mL and mean ALT was 123 U/L. There was an overall higher proportion in the tenofovir disoproxil fumarate group with HBV DNA <400 copies/mL (69 IU/mL) and ALT normalization rate at Week 48 compared to the placebo group (Table 24). There was no difference between treatment groups in those who achieved HBeAg loss or HBeAg seroconversion.Table 24 Outcomes of Randomized Treatment (Trial 144) in Children 2 Years to <12 Years of Age
Endpoint at Week 48
Tenofovir Disoproxil Fumarate N=60
Placebo N=29
HBV DNA <400 copies/mL (69 IU/ml)
46/60 (77%)
2/29 (7%)
ALT Normalizationa
38/58 (66%)
4/27 (15%)
HBeAg lossb
17/56 (30%)
8/29 (28%)
HBeAg seroconversionb
14/56 (25%)
7/29 (24%)
a. Normal ALT was defined as ≤ 34 U/L for females 2-15 years or males 1-9 years old, and ≤ 43 U/L for males 10-15 years. The ALT Normalization analysis excluded 4 treated subjects who had normal ALT at baseline.
b. The analysis excluded 4 subjects who were HBeAg negative and HBeAb positive at baseline.
In Trials 115 and 144, sequencing data from paired baseline and on treatment HBV isolates from subjects who received tenofovir disoproxil fumarate were available for 14 of 15 subjects who had plasma HBV DNA ≥400 copies/mL. No amino acid substitutions associated with resistance to tenofovir disoproxil fumarate were identified in these isolates by Week 72 (Trial 115) or Week 48 (Trial 144). -
16 HOW SUPPLIED/STORAGE AND HANDLINGTenofovir disoproxil fumarate tablets are available in bottles containing 30 tablets with child-resistant closure as follows: • 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil) ...
Tenofovir disoproxil fumarate tablets are available in bottles containing 30 tablets with child-resistant closure as follows:
• 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil): tablets are blue coloured, oval shaped, biconvex, film coated tablets are debossed with 'CL 77' on one side and plain on the other side, and are availableCartons of 30 film coated tablets (10 film coated tablets per blister pack x 3), NDC 0904-6821-04
Store below 30°C (86° F).
• Keep the bottle tightly closed.
Close
• Dispense only in original container.
• Do not use if seal over bottle opening is broken or missing. -
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Severe Acute Exacerbation of Hepatitis B in Patients Infected with HBV - Inform ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Severe Acute Exacerbation of Hepatitis B in Patients Infected with HBV
Inform patients that severe acute exacerbations of hepatitis B have been reported in patients infected with hepatitis B virus (HBV) and have discontinued tenofovir disoproxil fumarate tablets. Advise patients not to discontinue tenofovir disoproxil fumarate tablets without first informing their healthcare provider. All patients should be tested for HBV infection before or when starting tenofovir disoproxil fumarate tablets and those who are infected with HBV need close medical follow-up for several months after stopping tenofovir disoproxil fumarate tablets to monitor for exacerbations of hepatitis [see Warnings and Precautions (5.1)].New Onset or Worsening Renal Impairment
Inform patients that renal impairment, including cases of acute renal failure and Fanconi syndrome, has been reported in association with the use of tenofovir disoproxil fumarate tablets. Advise patients to avoid tenofovir disoproxil fumarate tablets with concurrent or recent use of a nephrotoxic agent (e.g., high-dose or multiple NSAIDs) [see Warnings and Precautions (5.2)]. The dosing interval of tenofovir disoproxil fumarate tablets may need adjustment in HIV-1 infected patients with renal impairment.Immune Reconstitution Syndrome
Inform patients that in some patients with advanced HIV infection (AIDS) signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started. It is believed that these symptoms are due to an improvement in the body’s immune response, enabling the body to fight infections that may have been present with no obvious symptoms. Advise patients to inform their healthcare provider immediately of any symptoms of infection [see Warnings and Precautions (5.4)].Bone Loss and Mineralization Defects
Inform patients that decreases in bone mineral density have been observed with the use of tenofovir disoproxil fumarate tablets. Consider bone monitoring in patients who have a history of pathologic bone fracture or at risk for osteopenia [see Warnings and Precautions (5.5)].Lactic Acidosis and Severe Hepatomegaly
Inform patients that lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported. Treatment with tenofovir disoproxil fumarate tablets should be suspended in any patient who develops clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.6)].Drug Interactions
Advise patients that tenofovir disoproxil fumarate tablets may interact with many drugs; therefore, advise patients to report to their healthcare provider the use of any other medication, including other HIV drugs and drugs for treatment of hepatitis C virus [see Warnings and Precautions (5.7) and Drug Interactions (7)].
Dosing Recommendations
Inform patients that it is important to take tenofovir disoproxil fumarate tablets on a regular dosing schedule with or without food and to avoid missing doses as it can result in development of resistance [see Dosage and Administration (2)].Pregnancy Registry
Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant women exposed to tenofovir disoproxil fumarate tablets. [see Use in Specific Populations (8.1)].Lactation
Instruct mothers not to breastfeed if they are taking tenofovir disoproxil fumarate tablets for the treatment of HIV-1 infection because of the risk of passing the HIV-1 virus to the baby [see Use in Specific Populations (8.2)].Treatment Duration
Advise patients that in the treatment of chronic hepatitis B, the optimal duration of treatment is unknown. The relationship between response and long-term prevention of outcomes such as hepatocellular carcinoma is not known.All other trademarks referenced herein are the property of their respective owners.
Manufactured for :
Macleods Pharma USA, Inc.
Princeton, NJ 08540Manufactured by:
Macleods Pharmaceuticals Limited, INDIAPackaged and Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268 USA
Refer to package label for Distributor's NDC Number
Revised: November 2022
Close -
PATIENT INFORMATIONTenofovir Disoproxil Fumarate Tablets - (ten-OF-oh-vir DYE-soe-PROX-il FUE-ma-rate) Read this Patient Information before you start taking tenofovir disoproxil fumarate tablets and each time ...
Tenofovir Disoproxil Fumarate Tablets
(ten-OF-oh-vir DYE-soe-PROX-il FUE-ma-rate)Read this Patient Information before you start taking tenofovir disoproxil fumarate tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment.
What is the most important information I should know about tenofovir disoproxil fumarate tablets?
Tenofovir disoproxil fumarate tablets can cause serious side effects, including:• Worsening of Hepatitis B virus infection (HBV). Your healthcare provider will test you for HBV and HIV before starting treatment with tenofovir disoproxil fumarate tablets. If you have HBV infection and take tenofovir disoproxil fumarate tablets your HBV may get worse (flare-up) if you stop taking tenofovir disoproxil fumarate tablets. A “flare-up” is when your HBV infection suddenly returns in a worse way than before.
• Do not run out of tenofovir disoproxil fumarate tablets. Refill your prescription or talk to your healthcare provider before your tenofovir disoproxil fumarate tablets are all gone.
• Do not stop taking tenofovir disoproxil fumarate tablets without first talking to your healthcare provider.
• If you stop taking tenofovir disoproxil fumarate tablets, your healthcare provider will need to check your health often and do blood tests regularly to check your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking tenofovir disoproxil fumarate tablets.For more information about side effects, see “What are the possible side effects of tenofovir disoproxil fumarate tablets?”
What are tenofovir disoproxil fumarate tablets?
Tenofovir disoproxil fumarate tablets are a prescription medicine that is used to:
• treat HIV-1 infection when used with other anti-HIV-1 medicines in adults and children 2 years of age and older who weigh at least 22 pounds (10 kg). HIV is the virus that causes AIDS (Acquired Immune Deficiency Syndrome).
• treat HBV infection in adults and children 2 years of age and older who weigh at least 22 pounds (10 kg). It is not known if tenofovir disoproxil fumarate tablets are safe and effective in children under 2 years of age.
What should I tell my healthcare provider before taking tenofovir disoproxil fumarate tablets?
Before you take tenofovir disoproxil fumarate tablets, tell your healthcare provider about all of your medical conditions, including if you:
• have liver problems, including HBV infection
• have kidney problems or receive kidney dialysis treatment
• have bone problems
• have HIV infection
• are pregnant or plan to become pregnant. Tell your healthcare provider if you become pregnant during treatment with tenofovir disoproxil fumarate tablets.
Pregnancy Registry. There is a pregnancy registry for women who take tenofovir disoproxil fumarate tablets during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
• are breastfeeding or plan to breastfeed. Tenofovir disoproxil fumarate can pass to your baby in your breast milk.
• Do not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
• If you take tenofovir disoproxil fumarate tablets for treatment of HBV infection, talk with your healthcare provider about the best way to feed your baby.Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Some medicines may interact with tenofovir disoproxil fumarate tablets. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
• You can ask your healthcare provider or pharmacist for a list of medicines that interact with tenofovir disoproxil fumarate tablets.
• Do not start a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take tenofovir disoproxil fumarate tablets with other medicines.
How should I take tenofovir disoproxil fumarate tablets?
• Take tenofovir disoproxil fumarate tablets exactly as your healthcare provider tells you to take it.
• Do not change your dose or stop taking tenofovir disoproxil fumarate tablets without first talking with your healthcare provider. Stay under a healthcare provider’s care when taking tenofovir disoproxil fumarate tablets.
• Take tenofovir disoproxil fumarate tablets at the same time every day.
• For adults and children 2 years of age and older who weigh at least 77 pounds (35 kg), the usual dose of tenofovir disoproxil fumarate tablet is one 300 mg tablet each day.
• For children 2 years of age and older who weigh between 37 pounds (17 kg) and 77 pounds (35 kg), your healthcare provider will prescribe the right dose of tenofovir disoproxil fumarate tablets based on your child’s body weight.
• Tell your healthcare provider if you or your child has problems with swallowing tablets.
• Take tenofovir disoproxil fumarate tablets by mouth, with or without food.
•Do not miss a dose of tenofovir disoproxil fumarate tablets. Missing a dose lowers the amount of medicine in your blood. Refill your tenofovir disoproxil fumarate tablets prescription before you run out of medicine.
• If you take too much tenofovir disoproxil fumarate tablets, call your local poison control center or go right away to the nearest hospital emergency room.What are the possible side effects of tenofovir disoproxil fumarate tablets?
Tenofovir disoproxil fumarate tablets may cause serious side effects, including:
• See “What is the most important information I should know about tenofovir disoproxil fumarate tablets?”
• New or worse kidney problems, including kidney failure. Your healthcare provider should do blood and urine tests to check your kidneys before you start and during treatment with tenofovir disoproxil fumarate tablets. Your healthcare provider may tell you to take tenofovir disoproxil fumarate tablets less often, or to stop taking tenofovir disoproxil fumarate tablets if you get new or worse kidney problems.
• Changes in your immune system (Immune Reconstitution Syndrome) can happen when an HIV-1 infected person starts taking HIV medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your tenofovir disoproxil fumarate tablets for the treatment of HIV-1 infection.
• Bone problems can happen in some children or adults who take tenofovir disoproxil fumarate tablets. Bone problems include bone pain, or softening or thinning of bones, which may lead to fractures. Your healthcare provider may need to do tests to check your bones or your child’s bones.
• Too much lactic acid in your blood (lactic acidosis). Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms: weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
• Severe liver problems. In rare cases, severe liver problems can happen that can lead to death. Tell your healthcare provider right away if you get these symptoms: skin or the white part of your eyes turns yellow, dark “tea-colored” urine, light-colored stools, loss of appetite for several days or longer, nausea, or stomach-area pain.
The most common side effects in all people taking tenofovir disoproxil fumarate tablets are:- •
- nausea
- •
- pain
- •
- rash
- •
- depression
- •
- diarrhea
- •
- weakness
- •
- headache
In some people with advanced HBV-infection, other common side effects may include:
- •
- fever
- •
- stomach-area pain
- •
- itching
- •
- dizziness
- •
- vomiting
- •
- sleeping problems
These are not all the possible side effects of tenofovir disoproxil fumarate tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store tenofovir disoproxil fumarate tablets?
• Store below 30°C (86° F).
• Keep tenofovir disoproxil fumarate tablets in the original container.
• Keep the bottle tightly closed.
• Do not use tenofovir disoproxil fumarate tablets if the seal over the bottle opening is broken or missing.
Keep tenofovir disoproxil fumarate tablets and all medicines out of the reach of children.General information about the safe and effective use of tenofovir disoproxil fumarate tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use tenofovir disoproxil fumarate tablets for a condition for which it was not prescribed. Do not give tenofovir disoproxil fumarate tablets to other people, even if they have the same condition you have. It may harm them. You can ask your pharmacist or healthcare provider for information about tenofovir disoproxil fumarate tablets that is written for health professionals.
A vaccine is available to protect people at risk for becoming infected with HBV. You can ask your healthcare provider for information about this vaccine.What are the ingredients in tenofovir disoproxil fumarate tablets?
Active Ingredient: tenofovir disoproxil fumarate
Inactive Ingredients:
Tenofovir disoproxil fumarate tablets: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.Tablet Coating:
Opadry 30K505001 which contains FD&C blue #2 , aluminum lake, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.All other trademarks referenced herein are the property of their respective owners.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured for :
Macleods Pharma USA, Inc.
Princeton, NJ 08540Manufactured by:
Macleods Pharmaceuticals Limited, INDIAPackaged and Distributed by:
MAJOR® PHARMACEUTICALS
Indianapolis, IN 46268 USA
Refer to package label for Distributor's NDC Number
Revised: November 2022
Close -
Package/Label Display Panel MAJOR® NDC 0904-6821-04 - Unit Dose - Tenofovir Disoproxil - Fumarate - Tablets - 300 mg - Pharmacist: Dispense with Patient - Information Leaflet - 30 TABLETS (3 x 10) Rx only
MAJOR®
NDC 0904-6821-04
Unit Dose
Tenofovir Disoproxil
Fumarate
Tablets
300 mg
Pharmacist: Dispense with Patient
Information Leaflet
30 TABLETS (3 x 10)
Rx only
Close -
INGREDIENTS AND APPEARANCEProduct Information
TENOFOVIR DISOPROXIL FUMARATE tenofovir disoproxil fumarate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0904-6821(NDC:33342-096) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TENOFOVIR DISOPROXIL FUMARATE (UNII: OTT9J7900I) (TENOFOVIR ANHYDROUS - UNII:W4HFE001U5) TENOFOVIR DISOPROXIL FUMARATE 300 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) FD&C BLUE NO. 2 ALUMINUM LAKE (UNII: 4AQJ3LG584) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color BLUE Score no score Shape OVAL (oval shaped,biconvex) Size 17mm Flavor Imprint Code CL77 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-6821-04 30 in 1 CARTON 01/26/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203232 01/26/2018
CloseLabeler - Major Pharmaceuticals (191427277)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
TENOFOVIR DISOPROXIL FUMARATE tablet, film coated
Get Label RSS Feed for this Drug
TENOFOVIR DISOPROXIL FUMARATE tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=da78a5d3-f5c6-47fe-b593-3252704c225d
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
TENOFOVIR DISOPROXIL FUMARATE tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0904-6821-04 |


