Label: CVS DECOLORIZED IODINE- alcohol liquid
- NDC Code(s): 69842-207-92
- Packager: CVS Pharmacy
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredient
- Other information
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS DECOLORIZED IODINE
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-207 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.48 mL in 1 mL Inactive Ingredients Ingredient Name Strength AMMONIA (UNII: 5138Q19F1X) IODINE (UNII: 9679TC07X4) POTASSIUM IODIDE (UNII: 1C4QK22F9J) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-207-92 59 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/01/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/01/2008 Labeler - CVS Pharmacy (062312574) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 analysis(69842-207) , manufacture(69842-207) , pack(69842-207) , label(69842-207)



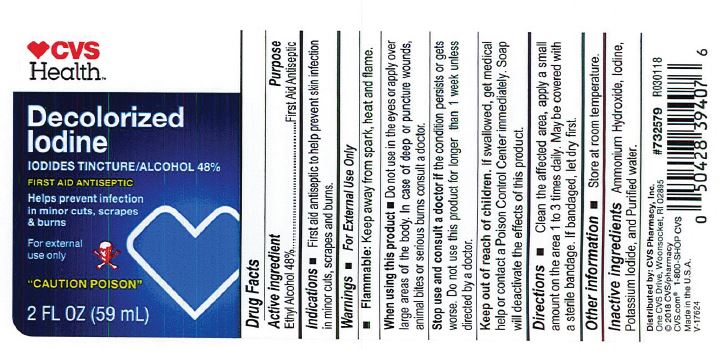



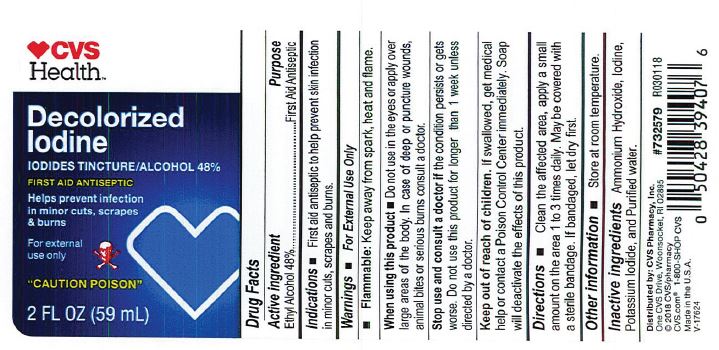 Decolorized
Decolorized
