Label: METRONIDAZOLE gel
- NDC Code(s): 59651-813-60
- Packager: Aurobindo Pharma Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use METRONIDAZOLE GEL, 1% safely and effectively. See full prescribing information for METRONIDAZOLE GEL, 1%. METRONIDAZOLE gel, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEMetronidazole gel, 1% is indicated for the topical treatment of inflammatory lesions of rosacea.
-
2 DOSAGE AND ADMINISTRATIONCleanse treated areas before the application of metronidazole gel. Apply and rub in a thin film of metronidazole gel once daily to affected area(s). Cosmetics may be applied after the application ...
-
3 DOSAGE FORMS AND STRENGTHSGel, 1%. Metronidazole gel, USP is a clear colorless to pale yellow gel. Each gram of metronidazole gel, USP contains 10 mg (1%) of metronidazole USP.
-
4 CONTRAINDICATIONSMetronidazole gel is contraindicated in patients with a history of hypersensitivity to metronidazole or to any other ingredient in the formulation.
-
5 WARNINGS AND PRECAUTIONS5.1 Neurologic Disease - Peripheral neuropathy, characterized by numbness or paresthesia of an extremity, has been reported in patients treated with systemic metronidazole. Peripheral neuropathy ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Neurologic Disease [see Warnings and Precautions (5.1)] Contact Dermatitis [see Warnings and ...
-
7 DRUG INTERACTIONSOral metronidazole has been reported to potentiate the anticoagulant effect of coumarin and warfarin, resulting in a prolongation of prothrombin time. Use caution when prescribing for patients who ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data have not established an association between metronidazole use during pregnancy and major birth defects, miscarriage or other adverse maternal or ...
-
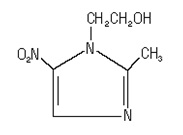
11 DESCRIPTIONMetronidazole gel USP 1% is a nitroimidazole for topical use. Metronidazole gel, USP is a clear colorless to pale yellow gel. Each gram contains 10 mg of metronidazole USP. Chemically ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of metronidazole in the treatment of rosacea is unknown. 12.2 Pharmacodynamics - The pharmacodynamics of metronidazole in association with ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Metronidazole has shown evidence of carcinogenic activity in studies involving chronic oral administration in mice and rats, but not in ...
-
14 CLINICAL STUDIESIn a randomized, vehicle-controlled trial, 746 subjects with rosacea were treated with metronidazole gel or vehicle once daily for 10 weeks. Most subjects had a disease severity score of 3 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - Metronidazole gel USP, 1% is clear colorless to pale yellow in color, and supplied as follows: 60 gram tube NDC 59651-813-60 - Storage and ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Administration Instructions - Use as directed. Avoid contact with the eyes [see Warnings and Precautions ...
-
SPL UNCLASSIFIED SECTIONPATIENT INFORMATION - Metronidazole (met" roe nid' a zole) Gel, USP - Important: Metronidazole gel is for use on the skin only (topical use). Do not use metronidazole gel in your mouth ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 60 g Tube LabelNDC 59651-813-60 - Metronidazole Gel, USP - 1% For topical use only - AUROBINDO Rx only NET WT. 60 g
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL- 60 g Tube CartonNDC 59651-813-60 - Metronidazole Gel, USP - 1% For topical use only - AUROBINDO Rx only NET WT. 60 g
-
INGREDIENTS AND APPEARANCEProduct Information