Label: ARGATROBAN injection, solution
- NDC Code(s): 55150-241-01, 55150-241-10
- Packager: Eugia US LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ARGATROBAN IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for ARGATROBAN IN SODIUM CHLORIDE INJECTION.
ARGATROBAN in sodium chloride injection, for intravenous use
Initial U.S. Approval: 2000INDICATIONS AND USAGE
Argatroban in sodium chloride injection is a direct thrombin inhibitor indicated:
DOSAGE AND ADMINISTRATION
- Argatroban 50 mg in 50 mL aqueous sodium chloride solution (1 mg/mL) is intended for administration to adult patients (2.1)
Heparin-Induced Thrombocytopenia (2.2)
The dose for heparin-induced thrombocytopenia without hepatic impairment is 2 mcg/kg/min administered as a continuous infusion (2.2)
Percutaneous Coronary Intervention (2.3)
The dose for patients with or at risk for heparin-induced thrombocytopenia undergoing percutaneous coronary intervention is started at 25 mcg/kg/min and a bolus of 350 mcg/kg administered via a large bore intravenous line over 3 to 5 minutes (2.3)DOSAGE FORMS AND STRENGTHS
Injection: 50 mg/50 mL (1 mg/mL) clear solution in single-dose vial (3)
WARNINGS AND PRECAUTIONS
- Risk of hemorrhage: Hemorrhage at any site can occur (unexplained fall in hematocrit of blood pressure or other unexplained symptom may indicate hemorrhage). Use with caution in patients at risk, including those receiving antiplatelet agents, thrombolytics, or other anticoagulants. (5.1)
- Use in hepatic impairment: Adjust starting dose and titrate carefully in patients with HIT who have moderate or severe hepatic impairment. Avoid use in PCI in patients with clinically significant hepatic impairment (5.2)
ADVERSE REACTIONS
- HIT patients: The most common (> 5%) adverse reactions were dyspnea, hypotension, fever, diarrhea, sepsis, and cardiac arrest (6.1)
- PCI patients: The most common (> 5%) adverse reactions were chest pain, hypotension, back pain, nausea, vomiting and headache (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Eugia US LLC at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Heparin-Induced Thrombocytopenia
1.2 Percutaneous Coronary Intervention
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Intravenous Administration
2.2 Dosing in Patients with Heparin-Induced Thrombocytopenia
2.3 Dosing in Patients Undergoing Percutaneous Coronary Interventions
2.4 Dosing in Patients with Hepatic Impairment
2.5 Conversion to Oral Anticoagulant Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hemorrhage
5.2 Use in Hepatic Impairment
5.3 Laboratory Tests
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Heparin
7.2 Oral Anticoagulant Agents
7.3 Aspirin/Acetaminophen
7.4 Thrombolytic Agents
7.5 Glycoprotein IIb/IIIa Antagonists
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Heparin-Induced Thrombocytopenia
14.2 Percutaneous Coronary Intervention (PCI) Patients with or at Risk for HIT
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Intravenous Administration
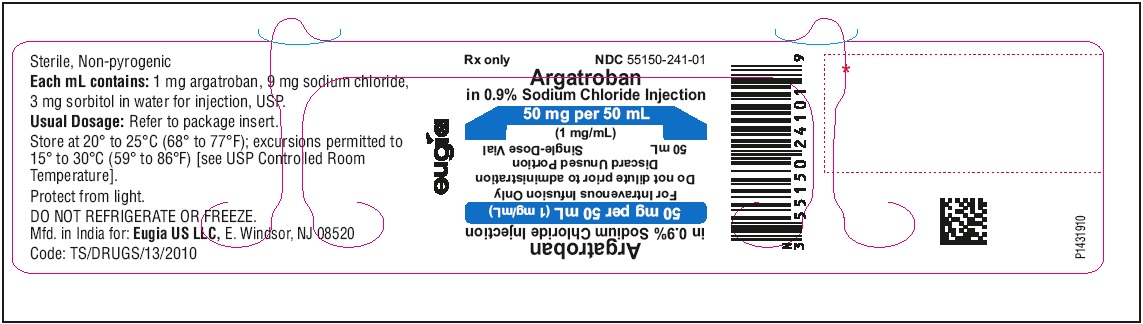
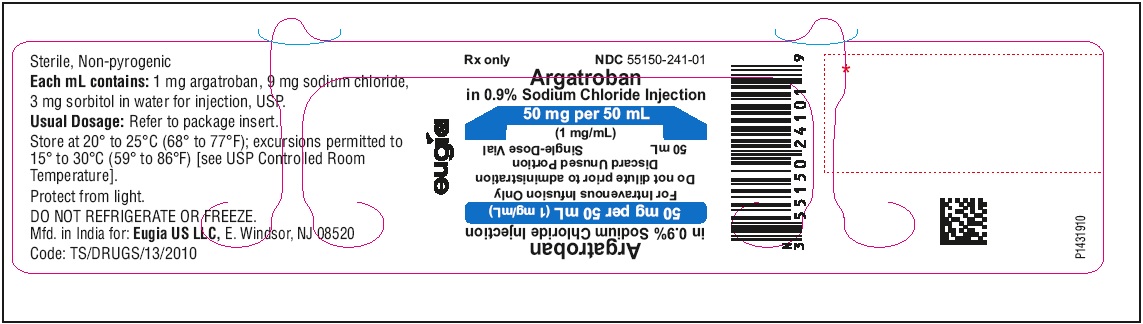
Each 50 mL glass vial contains 50 mg of argatroban (1 mg/mL); and, as supplied, is ready for intravenous infusion. Dilution is not required.
Argatroban in sodium chloride injection is a clear, colorless to pale yellow solution. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Do not use if the solution is cloudy, contains precipitates, or if the cap/over seal is not intact.
Vial may be inverted for use with a medical infusion set.2.2 Dosing in Patients with Heparin-Induced Thrombocytopenia
Initial Dosage:
Before administering Argatroban in sodium chloride injection, discontinue heparin therapy and obtain a baseline aPTT. The recommended initial dose of Argatroban in sodium chloride injection for adult patients without hepatic impairment is 2 mcg/kg/min, administered as a continuous infusion (see Table 1).Table 1. 1. with or without thrombosis Recommended Doses and Infusion Rates for 2 mcg/kg/min Dose of Argatroban in Sodium Chloride Injection for Patients With HIT1 and Without Hepatic Impairment (1 mg/mL Concentration)
Body Weight
(kg)
Dose
(mcg/min)
Infusion Rate
(mL/hr)
50
100
6
60
120
7
70
140
8
80
160
10
90
180
11
100
200
12
110
220
13
120
240
14
130
260
16
140
280
17
Monitoring Therapy:
For use in HIT, therapy with Argatroban in sodium chloride injection is monitored using the aPTT with a target range of 1.5 to 3 times the initial baseline value (not to exceed 100 seconds). Tests of anticoagulant effects (including the aPTT) typically attain steady-state levels within 1 to 3 hours following initiation of Argatroban in sodium chloride injection. Check the aPTT 2 hours after initiation of therapy and after any dose change to confirm that the patient has attained the desired therapeutic range.
Dosage Adjustment:
After the initiation of Argatroban in sodium chloride injection, adjust the dose (not to exceed 10 mcg/kg/min) as necessary to obtain a steady-state aPTT in the target range [see Clinical Studies (14.1)].2.3 Dosing in Patients Undergoing Percutaneous Coronary Interventions
Initial Dosage:
Initiate an infusion of Argatroban in sodium chloride injection at 25 mcg/kg/min and administer a bolus of 350 mcg/kg via a large bore intravenous line over 3 to 5 minutes (see Table 2). Check an activated clotting time (ACT) 5 to 10 minutes after the bolus dose is completed. The PCI procedure may proceed if the ACT is greater than 300 seconds.
Dosage Adjustment:
If the ACT is less than 300 seconds, an additional intravenous bolus dose of 150 mcg/kg should be administered, the infusion dose increased to 30 mcg/kg/min, and the ACT checked 5 to 10 minutes later (see Table 2).
If the ACT is greater than 450 seconds, decrease the infusion rate to 15 mcg/kg/min, and check the ACT 5 to 10 minutes later (Table 3).
Continue titrating the dose until a therapeutic ACT (between 300 and 450 seconds) has been achieved; continue the same infusion rate for the duration of the PCI procedure.
In case of dissection, impending abrupt closure, thrombus formation during the procedure, or inability to achieve or maintain an ACT over 300 seconds, additional bolus doses of 150 mcg/kg may be administered and the infusion dose increased to 40 mcg/kg/min. Check the ACT after each additional bolus or change in the rate of infusion.Table 2. NOTE: 1 mg = 1,000 mcg; 1 kg = 2.2 lbs Recommended Starting and Maintenance Doses (Within the Target ACT Range) of Argatroban in Sodium Chloride Injection in Patients Undergoing PCI Without Hepatic Impairment
(1 mg/mL Concentration)
Body Weight
(kg)
Starting Bolus Dose
(350 mcg/kg)
Starting and Maintenance Continuous Infusion Dosing
For ACT 300 to 450 seconds
25 mcg/kg/min
Bolus Dose (mcg)
Bolus Volume (mL)
Continuous Infusion Dose (mcg/min)
Continuous Infusion Rate
(mL/hr)
50
17,500
18
1,250
75
60
21,000
21
1,500
90
70
24,500
25
1,750
105
80
28,000
28
2,000
120
90
31,500
32
2,250
135
100
35,000
35
2,500
150
110
38,500
39
2,750
165
120
42,000
42
3,000
180
130
45,500
46
3,250
195
140
49,000
49
3,500
210
Table 3. NOTE: 1 mg = 1,000 mcg; 1 kg = 2.2 lbs
1. Additional intravenous bolus dose of 150 mcg/kg should be administered if ACT less than 300 seconds.
2. No bolus dose is given if ACT greater than 450 secondsRecommended Dose Adjustments of Argatroban in Sodium Chloride Injection for Patients Outside of ACT Target Range Undergoing PCI Without Hepatic Impairment (1 mg/mL Concentration)
Body Weight
(kg)
If ACT
Less than 300 seconds
Dosage Adjustment1
30 mcg/kg/min
If ACT
Greater than 450 seconds
Dosage Adjustment2
15 mcg/kg/min
Additional Bolus Dose
(mcg)
Bolus Volume
(mL)
Continuous Infusion Dose
(mcg/min)
Continuous Infusion Rate
(mL/hr)
Continuous Infusion Dose
(mcg/min)
Continuous Infusion Rate
(mL/hr)
50
7,500
8
1,500
90
750
45
60
9,000
9
1,800
108
900
54
70
10,500
11
2,100
126
1,050
63
80
12,000
12
2,400
144
1,200
72
90
13,500
14
2,700
162
1,350
81
100
15,000
15
3,000
180
1,500
90
110
16,500
17
3,300
198
1,650
99
120
18,000
18
3,600
216
1,800
108
130
19,500
20
3,900
234
1,950
117
140
21,000
21
4,200
252
2,100
126
Monitoring Therapy:
For use in PCI, therapy with Argatroban in sodium chloride injection is monitored using ACT. Obtain ACTs before dosing, 5 to 10 minutes after bolus dosing, following adjustments in the infusion rate, and at the end of the PCI procedure. Obtain additional ACTs every 20 to 30 minutes during a prolonged procedure.
Continued Anticoagulation after PCI:
If a patient requires anticoagulation after the procedure, Argatroban in sodium chloride injection may be continued, but at a rate of 2 mcg/kg/min and adjusted as needed to maintain the aPTT in the desired range [see Dosage and Administration (2.2)].2.4 Dosing in Patients with Hepatic Impairment
For adult patients with HIT and moderate or severe hepatic impairment (based on Child-Pugh classification), an initial dose of 0.5 mcg/kg/min is recommended, based on the approximately 4-fold decrease in argatroban clearance relative to those with normal hepatic function. Monitor the aPTT closely, and adjust the dosage as clinically indicated.
Monitoring Therapy:
Achievement of steady state aPTT levels may take longer and require more dose adjustments in patients with hepatic impairment compared to patients with normal hepatic function.
For patients with hepatic impairment undergoing PCI and who have HIT or are at risk for HIT, carefully titrate Argatroban in sodium chloride injection until the desired level of anticoagulation is achieved. Use of Argatroban in sodium chloride injection in PCI patients with clinically significant hepatic disease or AST/ALT levels ≥3 times the upper limit of normal should be avoided [see Warnings and Precautions (5.2)].2.5 Conversion to Oral Anticoagulant Therapy
Initiating Oral Anticoagulant Therapy:
When converting patients from Argatroban in sodium chloride injection to oral anticoagulant therapy, consider the potential for combined effects on International Normalized Ratio (INR). To avoid prothrombotic effects and to ensure continuous anticoagulation when initiating warfarin, overlap Argatroban in sodium chloride injection and warfarin therapy. There are insufficient data available to recommend the duration of the overlap. Initiate therapy using the expected daily dose of warfarin. A loading dose of warfarin should not be used.
The relationship between INR and bleeding risk is altered when Argatroban in sodium chloride injection and warfarin are co-administered. The combination of Argatroban in sodium chloride injection and warfarin does not cause further reduction in the vitamin K–dependent factor Xa activity than that which is seen with warfarin alone. The relationship between INR obtained on combined therapy and INR obtained on warfarin alone is dependent on both the dose of Argatroban in sodium chloride injection and the thromboplastin reagent used. The INR value on warfarin alone (INRW) can be calculated from the INR value on combination Argatroban in sodium chloride injection and warfarin therapy [see Drug Interactions (7.2) and Clinical Pharmacology (12.2)].
Co-Administration of Warfarin and Argatroban in Sodium Chloride Injection at Doses Up to 2 mcg/kg/min:
Measure INR daily while Argatroban in sodium chloride injection and warfarin are co-administered. In general, with doses of Argatroban in sodium chloride injection up to 2 mcg/kg/min, Argatroban in sodium chloride injection can be discontinued when the INR is >4 on combined therapy. After Argatroban in sodium chloride injection is discontinued, repeat the INR measurement in 4 to 6 hours. If the repeat INR is below the desired therapeutic range, resume the infusion of Argatroban in sodium chloride injection and repeat the procedure daily until the desired therapeutic range on warfarin alone is reached.
Co-Administration of Warfarin and Argatroban in Sodium Chloride Injection at Doses Greater than 2 mcg/kg/min:
For doses greater than 2 mcg/kg/min, the relationship of INR between warfarin alone to the INR on warfarin plus Argatroban in sodium chloride injection is less predictable. In this case, in order to predict the INR on warfarin alone, temporarily reduce the dose of Argatroban in sodium chloride injection to a dose of 2 mcg/kg/min. Repeat the INR on Argatroban in sodium chloride injection and warfarin 4 to 6 hours after reduction of the Argatroban in sodium chloride injection dose and follow the process outlined above for administering Argatroban in sodium chloride injection at doses up to 2 mcg/kg/min. - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Argatroban in sodium chloride injection is contraindicated in:
- Patients with major bleeding, [see Warnings and Precautions (5.1)]
- Patients with a history of hypersensitivity to argatroban products. Airway, skin, and generalized hypersensitivity reactions have been reported [see Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hemorrhage
Hemorrhage can occur at any site in the body in patients receiving Argatroban in sodium chloride injection. An unexplained fall in hematocrit or hemoglobin or a fall in blood pressure may indicate hemorrhage. Intracranial and retroperitoneal hemorrhage [see Adverse Reactions (6.1)] have been reported. The risk of hemorrhage with Argatroban in sodium chloride injection may be increased in severe hypertension; immediately following lumbar puncture; spinal anesthesia; major surgery (especially involving the brain, spinal cord, or eye); hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders and gastrointestinal lesions such as ulcerations.
Concomitant use of Argatroban in sodium chloride injection with antiplatelet agents, thrombolytics, and other anticoagulants may increase the risk of bleeding.5.2 Use in Hepatic Impairment
When administering Argatroban in sodium chloride injection to patients with hepatic impairment, start with a lower dose and carefully titrate until the desired level of anticoagulation is achieved. Achievement of steady state aPTT levels may take longer and require more Argatroban in sodium chloride injection dose adjustments in patients with hepatic impairment compared to patients with normal hepatic function [see Use in Specific Populations (8.6)]. Also, upon cessation of Argatroban in sodium chloride injection in the hepatically impaired patient, full reversal of anticoagulant effects may require longer than 4 hours due to decreased clearance and increased elimination half-life of argatroban [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. Avoid the use of high doses of Argatroban in sodium chloride injection in patients undergoing PCI who have clinically significant hepatic disease or AST/ALT levels ≥3 times the upper limit of normal.
5.3 Laboratory Tests
Anticoagulation effects associated with Argatroban in sodium chloride injection at doses up to 40 mcg/kg/min correlate with increases of the activated partial thromboplastin time (aPTT). Although other global clot-based tests including prothrombin time (PT), the International Normalized Ratio (INR), and thrombin time (TT) are affected by Argatroban in sodium chloride injection, the therapeutic ranges for these tests have not been identified for Argatroban in sodium chloride injection therapy. In clinical trials in PCI, the activated clotting time (ACT) was used for monitoring argatroban anticoagulant activity during the procedure. The concomitant use of Argatroban in sodium chloride injection and warfarin results in prolongation of the PT and INR beyond that produced by warfarin alone [see Dosage and Administration (2.5) and Clinical Pharmacology (12.2)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Risk of Hemorrhage [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Adverse Reactions in Patients with HIT (With or Without Thrombosis)
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following safety information is based on all 568 patients treated with argatroban in Study 1 and Study 2. The safety profile of the patients from these studies is compared with that of 193 historical controls in which the adverse reactions were collected retrospectively. Adverse reactions are separated into hemorrhagic and non-hemorrhagic reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥2 g/dL, that led to a transfusion of ≥2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint. Minor bleeding was overt bleeding that did not meet the criteria for major bleeding.
Table 4 gives an overview of the most frequently observed hemorrhagic adverse reactions, presented separately by major and minor bleeding, sorted by decreasing occurrence among argatroban-treated patients with HIT (with or without thrombosis).
Table 4. DIC = disseminated intravascular coagulation.
BKA = below-the-knee amputation.
1. with or without thrombosis
2. Patients may have experienced more than 1 adverse reaction.
3. One patient experienced intracranial hemorrhage 4 days after discontinuation of argatroban and following therapy with urokinase and oral anticoagulation.
4. The historical control group consisted of patients with a clinical diagnosis of HIT (with or without thrombosis) that were considered eligible by an independent medical panel.
Major and Minor Hemorrhagic Adverse Reactions in Patients With HIT1
Major Hemorrhagic Reactions2
Argatroban-Treated Patients
(Study 1 and Study 2)
(n = 568)
%
Historical
Control4
(n = 193)
%
Overall bleeding
5.3
6.7
Gastrointestinal
2.3
1.6
Genitourinary and hematuria
0.9
0.5
Decrease in hemoglobin and hematocrit
0.7
0
Multisystem hemorrhage and DIC
0.5
1
Limb and BKA stump
0.5
0
Intracranial hemorrhage
03
0.5
Minor Hemorrhagic Reactions2
Argatroban-Treated Patients
(Study 1 and Study 2)
(n = 568)
%
Historical
Control4
(n = 193)
%
Gastrointestinal
14.4
18.1
Genitourinary and hematuria
11.6
0.8
Decrease in hemoglobin and hematocrit
10.4
0
Groin
5.4
3.1
Hemoptysis
2.9
0.8
Brachial
2.4
0.8
Table 5 gives an overview of the most frequently observed non-hemorrhagic reactions sorted by decreasing frequency of occurrence (≥2%) among argatroban-treated HIT/HITTS patients.
Table 5. 1. Patients may have experienced more than 1 adverse reaction.
2. With or without thrombosis
3. The historical control group consisted of patients with a clinical diagnosis of HIT (with or without thrombosis) that were considered eligible by an independent medical panelNon-hemorrhagic Adverse Reactions in Patients1 With HIT2
Argatroban-Treated Patients
(Study 1 and Study 2)
(n = 568)
%
Historical
Control3
(n = 193)
%
Dyspnea
8.1
8.8
Hypotension
7.2
2.6
Fever
6.9
2.1
Diarrhea
6.2
1.6
Sepsis
6.0
12.4
Cardiac arrest
5.8
3.1
Nausea
4.8
0.5
Ventricular tachycardia
4.8
3.1
Pain
4.6
3.1
Urinary tract infection
4.6
5.2
Vomiting
4.2
0
Infection
3.7
3.6
Pneumonia
3.3
9.3
Atrial fibrillation
3.0
11.4
Coughing
2.8
1.6
Abnormal renal function
2.8
4.7
Abdominal pain
2.6
1.6
Cerebrovascular disorder
2.3
4.1
Adverse Reactions in Patients with or at Risk for HIT Patients Undergoing PCI
The following safety information is based on 91 patients initially treated with argatroban and 21 patients subsequently re-exposed to argatroban for a total of 112 PCIs with argatroban anticoagulation. Adverse reactions are separated into hemorrhagic (Table 6) and non-hemorrhagic (Table 7) reactions.
Major bleeding was defined as bleeding that was overt and associated with a hemoglobin decrease ≥5 g/dL, that led to a transfusion of ≥2 units, or that was intracranial, retroperitoneal, or into a major prosthetic joint.
The rate of major bleeding reactions in patients treated with argatroban in the PCI trials was 1.8%.
Table 6. CABG = coronary artery bypass graft.
1. Patients may have experienced more than 1 adverse reaction.
2. 91 patients who underwent 112 interventions.
Major and Minor Hemorrhagic Adverse Reactions in Patients With HIT Undergoing PCI
Major Hemorrhagic Reactions1
Argatroban-Treated Patients
(n = 112)2
%
Retroperitoneal
0.9
Gastrointestinal
0.9
Intracranial
0
Minor Hemorrhagic Reactions1
Argatroban-Treated Patients
(n = 112)2
%
Groin (bleeding or hematoma)
3.6
Gastrointestinal (includes hematemesis)
2.6
Genitourinary (includes hematuria)
1.8
Decrease in hemoglobin and/or hematocrit
1.8
CABG (coronary arteries)
1.8
Access site
0.9
Hemoptysis
0.9
Other
0.9
Table 7 gives an overview of the most frequently observed non-hemorrhagic adverse reactions (>2%), sorted by decreasing frequency of occurrence among argatroban-treated PCI patients.
Table 7. 1. Patients may have experienced more than 1 adverse reaction.
2. 91 patients who underwent 112 interventions.Non-hemorrhagic Adverse Reactions1 in Patients With HIT Undergoing PCI
Argatroban Procedures1
(n = 112)2
%
Chest pain
15.2
Hypotension
10.7
Back pain
8.0
Nausea
7.1
Vomiting
6.3
Headache
5.4
Bradycardia
4.5
Abdominal pain
3.6
Fever
3.6
Myocardial infarction
3.6
There were 22 serious adverse reactions in 17 PCI patients (19.6% in 112 interventions). Table 8 lists the serious adverse reactions occurring in argatroban-treated patients with or at risk for HIT undergoing PCI.
Table 8. 1. Individual adverse reactions may also have been reported elsewhere (see Table 6 and 7).
2. 91 patients underwent 112 procedures. Some patients may have experienced more than 1 adverse reaction.Serious Adverse Reactions in Patients With HIT Undergoing PCI1
Coded Term
Argatroban Procedures2
(n = 112)
Myocardial infarction
4 (3.5%)
Angina pectoris
2 (1.8%)
Coronary thrombosis
2 (1.8%)
Myocardial ischemia
2 (1.8%)
Occlusion coronary
2 (1.8%)
Chest pain
1 (0.9%)
Fever
1 (0.9%)
Retroperitoneal hemorrhage
1 (0.9%)
Aortic stenosis
1 (0.9%)
Arterial thrombosis
1 (0.9%)
Gastrointestinal hemorrhage
1 (0.9%)
Gastrointestinal disorder (GERD)
1 (0.9%)
Cerebrovascular disorder
1 (0.9%)
Lung edema
1 (0.9%)
Vascular disorder
1 (0.9%)
Intracranial Bleeding in Other Populations
Increased risks for intracranial bleeding have been observed in investigational studies of argatroban for other uses. In a study of patients with acute myocardial infarction receiving both argatroban and thrombolytic therapy (streptokinase or tissue plasminogen activator), the overall frequency of intracranial bleeding was 1% (8 out of 810 patients). Intracranial bleeding was not observed in 317 subjects or patients who did not receive concomitant thrombolysis [see Drug Interactions (7.4)].
The safety and effectiveness of Argatroban in sodium chloride injection for cardiac indications other than PCI in patients with HIT have not been established. Intracranial bleeding was also observed in a prospective, placebo-controlled study of argatroban in patients who had onset of acute stroke within 12 hours of study entry. Symptomatic intracranial hemorrhage was reported in 5 of 117 patients (4.3%) who received argatroban at 1 to 3 mcg/kg/min and in none of the 54 patients who received placebo. Asymptomatic intracranial hemorrhage occurred in 5 (4.3%) and 2 (3.7%) of the patients, respectively.
Allergic Reactions
One hundred fifty-six allergic reactions or suspected allergic reactions were observed in 1,127 individuals who were treated with argatroban in clinical pharmacology studies or for various clinical indications. About 95% (148/156) of these reactions occurred in patients who concomitantly received thrombolytic therapy (e.g., streptokinase) or contrast media.
Allergic reactions or suspected allergic reactions in populations other than patients with HIT (with or without thrombosis) include (in descending order of frequency):
• Airway reactions (coughing, dyspnea): 10% or more
• Skin reactions (rash, bullous eruption): 1 to <10%
• General reactions (vasodilation): 1 to 10%
Limited data are available on the potential formation of drug-related antibodies. Plasma from 12 healthy volunteers treated with argatroban over 6 days showed no evidence of neutralizing antibodies. No loss of anticoagulant activity was noted with repeated administration of argatroban to more than 40 patients.
-
7 DRUG INTERACTIONS
7.1 Heparin
If Argatroban in sodium chloride injection is to be initiated after cessation of heparin therapy, allow sufficient time for heparin’s effect on the aPTT to decrease prior to initiation of Argatroban in sodium chloride injection therapy.
7.2 Oral Anticoagulant Agents
Pharmacokinetic drug-drug interactions between Argatroban in sodium chloride injection and warfarin (7.5 mg single oral dose) have not been demonstrated. However, the concomitant use of Argatroban in sodium chloride injection and warfarin (5 to 7.5 mg initial oral dose, followed by 2.5 to 6 mg/day orally for 6 to 10 days) results in prolongation of the prothrombin time (PT) and International Normalized Ratio (INR) [see Dosage and Administration (2.5), Clinical Pharmacology (12.2)].
7.3 Aspirin/Acetaminophen
No drug-drug interactions have been demonstrated between Argatroban in sodium chloride injection and concomitantly administered aspirin or acetaminophen [see Clinical Pharmacology (12.3)].
7.4 Thrombolytic Agents
The safety and effectiveness of Argatroban in sodium chloride injection with thrombolytic agents have not been established [see Adverse Reactions (6.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Limited data from published literature and postmarketing reports do not suggest an association between Argatroban in sodium chloride injection and adverse fetal developmental outcomes. There are risks to the mother associated with untreated thrombosis in pregnancy and a risk of hemorrhage in the mother and fetus associated with use of anticoagulants (see Clinical Considerations). In animal reproduction studies, there was no evidence of adverse developmental outcomes with intravenous administration of argatroban during organogenesis in rats and rabbits at doses up to 0.3- and 0.2 times, respectively, the maximum recommended human dose (MHRD) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.Clinical Considerations
Disease-Associated Maternal and/or Embryo-Fetal Risk
Pregnancy confers an increased risk for thromboembolism that is higher for women with underlying thromboembolic disease and certain high risk pregnancy conditions. Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy.
Fetal/Neonatal Adverse Reactions
Use of anticoagulants, including Argatroban in sodium chloride injection, may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding [see Warnings and Precautions (5.1, 5.3)].
Labor or Delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. Pregnant women receiving Argatroban in sodium chloride injection should be carefully monitored for evidence of excessive bleeding or unexpected changes in coagulation parameters [see Warnings and Precautions (5.1, 5.3)].Data
Animal Data
Developmental studies performed in rats with argatroban at intravenous doses up to 27 mg/kg/day (0.3 times the maximum recommended human dose, based on body surface area) and in rabbits at intravenous doses up to 10.8 mg/kg/day (0.2 times the maximum recommended dose for humans, based on body surface area) have revealed no evidence of harm to the fetus.8.2 Lactation
Risk Summary
There are no data on the presence of argatroban in human milk, or its effects on milk production. Argatroban is present in rat milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s need for Argatroban in sodium chloride injection and any potential adverse effects on the breastfed child from Argatroban in sodium chloride injection or from the underlying maternal condition.
Data
Animal Data
Argatroban is detected in rat milk.8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Argatroban was studied among 18 seriously ill pediatric patients who required an alternative to heparin anticoagulation. Most patients were diagnosed with HIT or suspected HIT. Age ranges of patients were <6 months, n = 8; six months to <8 years, n = 6; 8 to 16 years, n = 4. All patients had serious underlying conditions and were receiving multiple concomitant medications. Thirteen patients received argatroban solely as a continuous infusion (no bolus dose). Dosing was initiated in the majority of these 13 patients at 1 mcg/kg/min and subsequently titrated as needed to achieve and maintain an aPTT of 1.5 to 3 times the baseline value. Most patients required multiple dose adjustments to maintain anticoagulation parameters within the desired range. During the 30-day study period, thrombotic events occurred during argatroban administration to two patients and following argatroban discontinuation in three other patients. Major bleeding occurred among two patients; one patient experienced an intracranial hemorrhage after 4 days of argatroban therapy in the setting of sepsis and thrombocytopenia and another patient experienced an intracranial hemorrhage after receiving argatroban for greater than 14 days. The study findings did not establish the safe and effective use of argatroban in pediatric patients and the dosing of 1 mcg/kg/min was not supported by the pharmacokinetic data described below.
Pediatric Pharmacokinetics (PK) and Pharmacodynamics (PD)
PK parameters of argatroban were characterized in population PK/PD analysis model with sparse data from 15 seriously ill pediatric patients. Argatroban clearance in these seriously ill pediatric patients (0.16 L/hr/kg) was 50% lower compared to argatroban clearance in healthy adults (0.31 L/hr/kg). Four pediatric patients with elevated bilirubin (secondary to cardiac complications or hepatic impairment) had, on average, 80% lower clearance (0.03 L/hr/kg) when compared to pediatric patients with normal bilirubin levels.
These PK/PD analysis models based on a goal of aPTT prolongation of 1.5 to 3 times the baseline value and avoidance of an aPTT >100 seconds for seriously ill pediatric patients with HIT/HITTS who require an alternative to heparin suggested the following:- For patients with normal hepatic function, a starting infusion rate of 0.75 mcg/kg/min may have comparable aPTT responses as a starting dose of 2 mcg/kg/min in healthy adults. Additionally, based on an evaluation of aPTT every two hours, increasing the dosage by 0.1 to 0.25 mcg/kg/min could achieve additional aPTT responses.
- For patients with hepatic impairment a starting infusion rate of 0.2 mcg/kg/min with increasing dosing by increments of 0.05 mcg/kg/min may have comparable argatroban exposure as expected with adult doses.
The safety and effectiveness of Argatroban in sodium chloride injection with the above dosing have not been adequately assessed in pediatric patients and the safety and effectiveness of Argatroban in sodium chloride injection is not established in pediatric patients. In addition, the described dosage did not take into account multiple factors that could affect the dosage such as current aPTT, target aPTT, and the clinical status of the patient.
8.5 Geriatric Use
Of the total number of subjects (1340) in clinical studies of argatroban, 35% were 65 and over. In the clinical studies of adult patients with HIT (with or without thrombosis), the effectiveness of argatroban was not affected by age. No trends were observed across age groups for both aPTT and the ACT. The safety analysis did suggest that older patients tended to have an increased incidence of adverse reactions compared to younger patients; however, older patients had increased underlying conditions, which may predispose them to adverse reactions. The studies were not sized appropriately to detect differences in safety between age groups.
8.6 Hepatic Impairment
Dose reduction and careful titration are required when administering Argatroban in sodium chloride injection to patients with hepatic impairment. Reversal of anticoagulation effect may be prolonged in this population [see Dosage and Administration (2.4), Warnings and Precautions (5.2), Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Excessive anticoagulation, with or without bleeding, may be controlled by discontinuing Argatroban in sodium chloride injection or by decreasing the Argatroban in sodium chloride injection dose. In clinical studies, anticoagulation parameters generally returned from therapeutic levels to baseline within 2 to 4 hours after discontinuation of the drug. Reversal of anticoagulant effect may take longer in patients with hepatic impairment.
No specific antidote to Argatroban in sodium chloride injection is available; if life-threatening bleeding occurs and excessive plasma levels of argatroban are suspected, discontinue Argatroban in sodium chloride injection immediately and measure aPTT and other coagulation parameters. When argatroban was administered as a continuous infusion (2 mcg/kg/min) prior to and during a 4-hour hemodialysis session, approximately 20% of argatroban was cleared through dialysis.
Single intravenous doses of argatroban at 200, 124, 150, and 200 mg/kg were lethal to mice, rats, rabbits, and dogs, respectively. The symptoms of acute toxicity were loss of righting reflex, tremors, clonic convulsions, paralysis of hind limbs, and coma. -
11 DESCRIPTION
Argatroban in sodium chloride injection is a synthetic direct thrombin inhibitor.
The chemical name for argatroban monohydrate is 1-[5-[(aminoiminomethyl)amino]-1-oxo-2-[[(1,2,3,4-tetrahydro-3-methyl-8-quinolinyl)sulfonyl]amino]pentyl]-4-methyl-2-piperidinecarboxylic acid, monohydrate. Argatroban has 4 asymmetric carbons. One of the asymmetric carbons has an R configuration (stereoisomer Type I) and an S configuration (stereoisomer Type II). Argatroban consists of a mixture of R and S stereoisomers at a ratio of approximately 65:35.
The molecular formula of argatroban is C23H36N6O5S•H2O. Its molecular weight is 526.66 g/mol. The structural formula is:
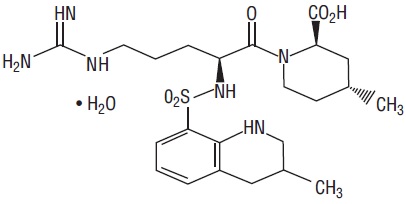
Argatroban in sodium chloride injection is a sterile, non-pyrogenic, clear, colorless to pale yellow solution. It is supplied in a clear glass single-dose vial containing 50 mg of argatroban in 50 mL sodium chloride solution. Each mL contains 1 mg argatroban, 9 mg sodium chloride, USP, 3 mg sorbitol, NF in water for injection, USP. The pH of the solution is between 4.0 to 7.5. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Argatroban in sodium chloride injection is a direct thrombin inhibitor that reversibly binds to the thrombin active site. Argatroban does not require the co-factor antithrombin III for antithrombotic activity. Argatroban exerts its anticoagulant effects by inhibiting thrombin-catalyzed or -induced reactions, including fibrin formation; activation of coagulation factors V, VIII, and XIII; activation of protein C; and platelet aggregation.
Argatroban inhibits thrombin with an inhibition constant (Ki) of 0.04 μM. At therapeutic concentrations, argatroban has little or no effect on related serine proteases (trypsin, factor Xa, plasmin, and kallikrein).
Argatroban is capable of inhibiting the action of both free and clot-associated thrombin.12.2 Pharmacodynamics
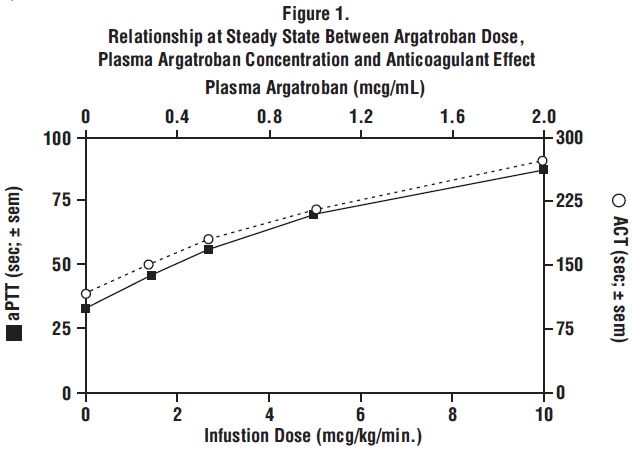
When argatroban is administered by continuous infusion, anticoagulant effects and plasma concentrations of argatroban follow similar, predictable temporal response profiles, with low intersubject variability. Immediately upon initiation of argatroban infusion, anticoagulant effects are produced as plasma argatroban concentrations begin to rise. Steady-state levels of both drug and anticoagulant effect are typically attained within 1 to 3 hours and are maintained until the infusion is discontinued or the dosage adjusted. Steady-state plasma argatroban concentrations increase proportionally with dose (for infusion doses up to 40 mcg/kg/min in healthy subjects) and are well correlated with steady-state anticoagulant effects. For infusion doses up to 40 mcg/kg/min, argatroban increases in a dose-dependent fashion, the activated partial thromboplastin time (aPTT), the activated clotting time (ACT), the prothrombin time (PT), the International Normalized Ratio (INR), and the thrombin time (TT) in healthy volunteers and cardiac patients. Representative steady-state plasma argatroban concentrations and anticoagulant effects are shown below for argatroban infusion doses up to 10 mcg/kg/min (see Figure 1).
Effect on International Normalized Ratio (INR): Because argatroban is a direct thrombin inhibitor, co-administration of argatroban and warfarin produces a combined effect on the laboratory measurement of the INR. However, concurrent therapy, compared to warfarin monotherapy, exerts no additional effect on vitamin K–dependent factor Xa activity.
The relationship between INR on co-therapy and warfarin alone is dependent on both the dose of argatroban and the thromboplastin reagent used. This relationship is influenced by the International Sensitivity Index (ISI) of the thromboplastin. Data for 2 commonly utilized thromboplastins with ISI values of 0.88 (Innovin, Dade) and 1.78 (Thromboplastin C Plus, Dade) are presented in Figure 2 for an argatroban dose of 2 mcg/kg/min. Thromboplastins with higher ISI values than shown result in higher INRs on combined therapy of warfarin and argatroban. These data are based on results obtained in normal individuals [see Dosage and Administration (2.5), Warnings and Precautions (5.3)].
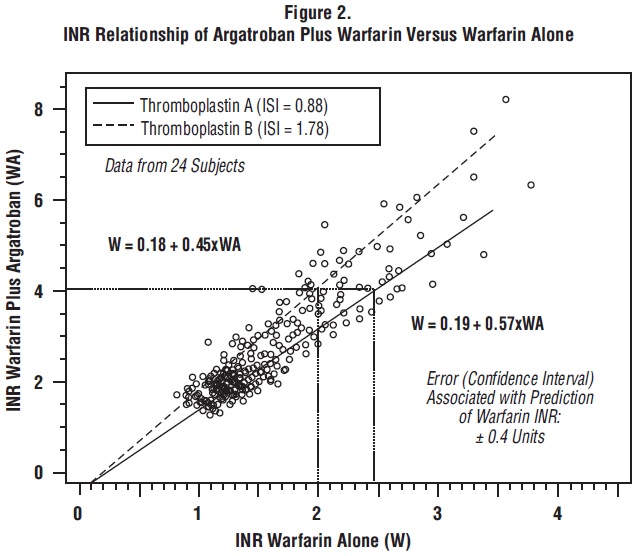
Figure 2 demonstrates the relationship between INR for warfarin alone and INR for warfarin co-administered with argatroban at a dose of 2 mcg/kg/min. To calculate INR for warfarin alone (INRW), based on INR for co-therapy of warfarin and argatroban (INRWA), when the argatroban dose is 2 mcg/kg/min, use the equation next to the appropriate curve. Example: At a dose of 2 mcg/kg/min and an INR performed with Thromboplastin A, the equation 0.19 + 0.57 (INRWA) = INRW would allow a prediction of the INR on warfarin alone (INRW). Thus, using an INRWA value of 4.0 obtained on combined therapy: INRW = 0.19 + 0.57 (4) = 2.47 as the value for INR on warfarin alone. The error (confidence interval) associated with a prediction is ± 0.4 units. Similar linear relationships and prediction errors exist for argatroban at a dose of 1 mcg/kg/min. Thus, for argatroban doses of 1 or 2 mcg/kg/min, INRW can be predicted from INRWA. For argatroban doses greater than 2 mcg/kg/min, the error associated with predicting INRW from INRWA is ± 1. Thus, INRW cannot be reliably predicted from INRWA at doses greater than 2 mcg/kg/min.12.3 Pharmacokinetics
Distribution:
Argatroban distributes mainly in the extra cellular fluid as evidenced by an apparent steady-state volume of distribution of 174 mL/kg (12.18 L in a 70 kg adult). Argatroban is 54% bound to human serum proteins, with binding to albumin and α1-acid glycoprotein being 20% and 34%, respectively.
Metabolism:
The main route of argatroban metabolism is hydroxylation and aromatization of the 3-methyltetrahydroquinoline ring in the liver. The formation of each of the 4 known metabolites is catalyzed in vitro by the human liver microsomal cytochrome P450 enzymes CYP3A4/5. The primary metabolite (M1) exerts 3- to 5-fold weaker anticoagulant effects than argatroban. Unchanged argatroban is the major component in plasma. The plasma concentrations of M1 range between 0% and 20% of that of the parent drug. The other metabolites (M2 to M4) are found only in very low quantities in the urine and have not been detected in plasma or feces. These data, together with the lack of effect of erythromycin (a potent CYP3A4/5 inhibitor) on argatroban pharmacokinetics, suggest that CYP3A4/5-mediated metabolism is not an important elimination pathway in vivo.
Total body clearance is approximately 5.1 mL/kg/min (0.31 L/kg/hr) for infusion doses up to 40 mcg/kg/min. The terminal elimination half-life of argatroban ranges between 39 and 51 minutes.
There is no interconversion of the 21–(R):21–(S) diastereoisomers. The plasma ratio of these diastereoisomers is unchanged by metabolism or hepatic impairment, remaining constant at 65:35 (± 2%).
Excretion:
Argatroban is excreted primarily in the feces, presumably through biliary secretion. In a study in which 14C-argatroban (5 mcg/kg/min) was infused for 4 hours into healthy subjects, approximately 65% of the radioactivity was recovered in the feces within 6 days of the start of infusion with little or no radioactivity subsequently detected. Approximately 22% of the radioactivity appeared in the urine within 12 hours of the start of infusion. Little or no additional urinary radioactivity was subsequently detected. Average percent recovery of unchanged drug, relative to total dose, was 16% in urine and at least 14% in feces.
Special Populations:
Hepatic Impairment: The dosage of Argatroban in sodium chloride injection should be decreased in patients with hepatic impairment [see Dosage and Administration (2.4) and Warnings and Precautions (5.2)]. Patients with hepatic impairment were not studied in percutaneous coronary intervention (PCI) trials. At a dose of 2.5 mcg/kg/min, hepatic impairment is associated with decreased clearance and increased elimination half-life of argatroban (to 1.9 mL/kg/min and 181 minutes, respectively, for patients with a Child-Pugh score >6).
Renal Impairment: No dosage adjustment is necessary in patients with renal dysfunction. The effect of renal disease on the pharmacokinetics of argatroban was studied in 6 subjects with normal renal function (mean Clcr = 95 ± 16 mL/min) and in 18 subjects with mild (mean Clcr = 64 ± 10 mL/min), moderate (mean Clcr = 41 ± 5.8 mL/min), and severe (mean Clcr = 5 ± 7 mL/min) renal impairment. The pharmacokinetics and pharmacodynamics of argatroban at dosages up to 5 mcg/kg/min were not significantly affected by renal dysfunction.
Use of argatroban was evaluated in a study of 12 patients with stable end-stage renal disease undergoing chronic intermittent hemodialysis. Argatroban was administered at a rate of 2 to 3 mcg/kg/min (begun at least 4 hours prior to dialysis) or as a bolus dose of 250 mcg/kg at the start of dialysis followed by a continuous infusion of 2 mcg/kg/min. Although these regimens did not achieve the goal of maintaining ACT values at 1.8 times the baseline value throughout most of the hemodialysis period, the hemodialysis sessions were successfully completed with both of these regimens. The mean ACTs produced in this study ranged from 1.39 to 1.82 times baseline, and the mean aPTTs ranged from 1.96 to 3.4 times baseline. When argatroban was administered as a continuous infusion of 2 mcg/kg/min prior to and during a 4-hour hemodialysis session, approximately 20% was cleared through dialysis.
Age, Gender: There are no clinically significant effects of age or gender on the pharmacokinetics or pharmacodynamics (e.g., aPTT) of argatroban in adults.
Drug-Drug Interactions:
Digoxin: In 12 healthy volunteers, intravenous infusion of argatroban (2 mcg/kg/min) over 5 days (study days 11 to 15) did not affect the steady-state pharmacokinetics of oral digoxin (0.375 mg daily for 15 days).
Erythromycin: In 10 healthy subjects, orally administered erythromycin (a potent inhibitor of CYP3A4/5) at 500 mg four times daily for 7 days had no effect on the pharmacokinetics of argatroban at a dose of 1 mcg/kg/min for 5 hours. These data suggest oxidative metabolism by CYP3A4/5 is not an important elimination pathway in vivo for argatroban.
Aspirin and Acetaminophen: Drug-drug interactions have not been demonstrated between argatroban and concomitantly administered aspirin (162.5 mg orally given 26 and 2 hours prior to initiation of argatroban 1 mcg/kg/min over 4 hours) or acetaminophen (1,000 mg orally given 12, 6, and 0 hours prior to, and 6 and 12 hours subsequent to, initiation of argatroban 1.5 mcg/kg/min over 18 hours). -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with argatroban have not been performed.
Argatroban was not genotoxic in the Ames test, the Chinese hamster ovary cell (CHO/HGPRT) forward mutation test, the Chinese hamster lung fibroblast chromosome aberration test, the rat hepatocyte, and WI-38 human fetal lung cell unscheduled DNA synthesis (UDS) tests, or the mouse micronucleus test.
Argatroban at intravenous doses up to 27 mg/kg/day (0.3 times the recommended maximum human dose based on body surface area) had no effect on fertility and reproductive function of male and female rats. -
14 CLINICAL STUDIES
14.1 Heparin-Induced Thrombocytopenia
The safety and efficacy of argatroban were evaluated in an historically controlled efficacy and safety study (Study 1) and a follow-on efficacy and safety study (Study 2). These studies were comparable with regard to study design, study objectives, dosing regimens as well as study outline, conduct, and monitoring.
In these studies, 568 adult patients were treated with argatroban and 193 adult patients made up the historical control group. Patients had a clinical diagnosis of heparin-induced thrombocytopenia, either without thrombosis (HIT) or with thrombosis (HITTS [heparin-induced thrombocytopenia and thrombosis syndrome]) and were males or non-pregnant females between the age of 18 and 80 years old. HIT/HITTS was defined by a fall in platelet count to less than 100,000/μL or a 50% decrease in platelets after the initiation of heparin therapy with no apparent explanation other than HIT. Patients with HITTS also had an arterial or venous thrombosis documented by appropriate imaging techniques or supported by clinical evidence such as acute myocardial infarction, stroke, pulmonary embolism, or other clinical indications of vascular occlusion. Patients who had documented histories of positive heparin-dependent antibody tests without current thrombocytopenia or heparin challenge (e.g., patients with latent disease) were also included if they required anticoagulation.
These studies did not include patients with documented unexplained aPTT >200% of control at baseline, documented coagulation disorder or bleeding diathesis unrelated to HIT, a lumbar puncture within the past 7 days or a history of previous aneurysm, hemorrhagic stroke, or a thrombotic stroke within the past 6 months unrelated to HIT.
The initial dose of argatroban was 2 mcg/kg/min. Two hours after the start of the argatroban infusion, an aPTT level was obtained and dose adjustments were made (up to a maximum of 10 mcg/kg/min) to achieve a steady-state aPTT value that was 1.5 to 3.0 times the baseline value, not to exceed 100 seconds. Overall the mean aPTT level for HIT and HITTS patients during the Argatroban infusion increased from baseline values of 34 and 38 seconds, respectively, to 62.5 and 64.5 seconds, respectively.
The primary efficacy analysis was based on a comparison of event rates for a composite endpoint that included death (all causes), amputation (all causes) or new thrombosis during the treatment and follow-up period (study days 0 to 37).
Secondary analyses included evaluation of the event rates for the components of the composite endpoint as well as time-to-event analyses.
In Study 1, a total of 304 patients were enrolled as follows: active HIT (n = 129), active HITTS (n = 144), or latent disease (n = 31). Among the 193 historical controls, 139 (72%) had active HIT, 46 (24%) had active HITTS, and 8 (4%) had latent disease. Within each group, those with active HIT and those with latent disease were analyzed together. Positive laboratory confirmation of HIT/HITTS by the heparin-induced platelet aggregation test or serotonin release assay was demonstrated in 174 of 304 (57%) argatroban-treated patients (i.e., in 80 with HIT or latent disease and 94 with HITTS) and in 149 of 193 (77%) historical controls (i.e., in 119 with HIT or latent disease and 30 with HITTS). The test results for the remainder of the patients and controls were either negative or not determined.
There was a significant improvement in the composite outcome in patients with HIT and HITTS treated with argatroban versus those in the historical control group (see Table 9). The components of the composite endpoint are shown in Table 9.Table 9. 1. Death (all cause), amputation (all cause), or new thrombosis within 37-day study period
2. Reported as the most severe outcome among the components of composite endpoint (severity ranking: death > amputation > new thrombosis); patients may have had multiple outcomes.Efficacy Results of Study 1: Composite Endpoint1 and Individual Components, Ranked by Severity2
HIT
HITTS
HIT/HITTS
Parameter, N (%)
Control n = 147
Argatroban n = 160
Control n = 46
Argatroban n = 144
Control
n = 193
Argatroban
n = 304
Composite Endpoint
57 (38.8)
41 (25.6)
26 (56.5)
63 (43.8)
83 (43.0)
104 (34.2)
Individual Components2:
Death
32 (21.8)
27 (16.9)
13 (28.3)
26 (18.1)
45 (23.3)
53 (17.4)
Amputation
3 (2.0)
3 (1.9)
4 (8.7)
16 (11.1)
7 (3.6)
19 (6.2)
New Thrombosis
22 (15.0)
11 (6.9)
9 (19.6)
21 (14.6)
31 (16.1)
32 (10.5)
Time-to-event analyses showed significant improvements in the time-to-first event in patients with HIT or HITTS treated with argatroban versus those in the historical control group. The between-group differences in the proportion of patients who remained free of death, amputation, or new thrombosis were statistically significant in favor of argatroban by these analyses.
A time-to-event analysis for the composite endpoint is shown in Figure 3 for patients with HIT and Figure 4 for patients with HITTS.
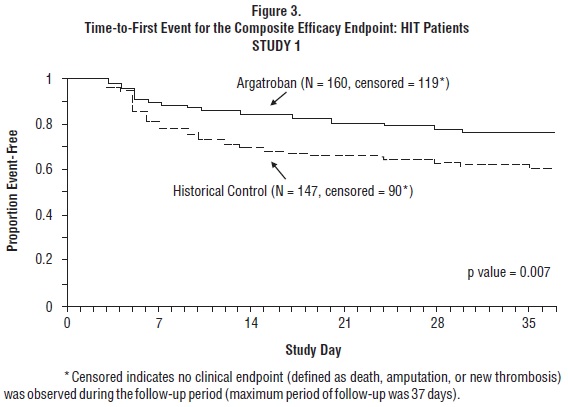
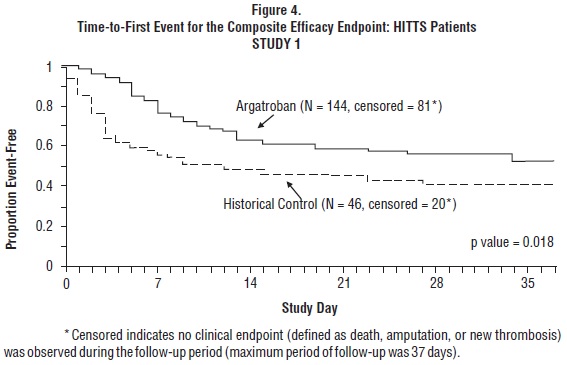
In Study 2, a total of 264 patients were enrolled as follows: HIT (n = 125) or HITTS (n = 139). There was a significant improvement in the composite efficacy outcome for argatroban-treated patients, versus the same historical control group from Study 1, among patients having HIT (25.6% vs. 38.8%), patients having HITTS (41.0% vs. 56.5%), and patients having either HIT or HITTS (33.7% vs. 43.0%). Time-to-event analyses showed significant improvements in the time-to-first event in patients with HIT or HITTS treated with argatroban versus those in the historical control group. The between-group differences in the proportion of patients who remained free of death, amputation, or new thrombosis were statistically significant in favor of argatroban.
Anticoagulant Effect:
In Study 1, the mean (± SE) dose of argatroban administered was 2.0 ± 0.1 mcg/kg/min in the HIT arm and 1.9 ± 0.1 mcg/kg/min in the HITTS arm. Seventy-six percent of patients with HIT and 81% of patients with HITTS achieved a target aPTT at least 1.5-fold greater than the baseline aPTT at the first assessment occurring on average at 4.6 hours (HIT) and 3.9 hours (HITTS) following initiation of argatroban therapy.
No enhancement of aPTT response was observed in subjects receiving repeated administration of argatroban.
Platelet Count Recovery:
In Study 1, 53% of patients with HIT and 58% of patients with HITTS, had a recovery of platelet count by Day 3. Platelet Count Recovery was defined as an increase in platelet count to >100,000/μL or to at least 1.5-fold greater than the baseline count (platelet count at study initiation) by Day 3 of the study.14.2 Percutaneous Coronary Intervention (PCI) Patients with or at Risk for HIT
In 3 similarly designed trials, argatroban was administered to 91 patients with current or previous clinical diagnosis of HIT or heparin-dependent antibodies, who underwent a total of 112 percutaneous coronary interventions (PCIs) including percutaneous transluminal coronary angioplasty (PTCA), coronary stent placement, or atherectomy.
Among the 91 patients undergoing their first PCI with argatroban, notable ongoing or recent medical history included myocardial infarction (n = 35), unstable angina (n = 23), and chronic angina (n = 34). There were 33 females and 58 males. The average age was 67.6 years (median 70.7, range 44 to 86), and the average weight was 82.5 kg (median 81.0 kg, range 49 to 141).
Twenty-one of the 91 patients had a repeat PCI using argatroban an average of 150 days after their initial PCI. Seven of 91 patients received glycoprotein IIb/IIIa inhibitors. Safety and efficacy were assessed against historical control populations who had been anticoagulated with heparin.
All patients received oral aspirin (325 mg) 2 to 24 hours prior to the interventional procedure. After venous or arterial sheaths were in place, anticoagulation was initiated with a bolus of argatroban of 350 mcg/kg via a large-bore intravenous line or through the venous sheath over 3 to 5 minutes. Simultaneously, a maintenance infusion of 25 mcg/kg/min was initiated to achieve a therapeutic activated clotting time (ACT) of 300 to 450 seconds. If necessary to achieve this therapeutic range, the maintenance infusion dose was titrated (15 to 40 mcg/kg/min) and/or an additional bolus dose of 150 mcg/kg could be given. Each patient’s ACT was checked 5 to 10 minutes following the bolus dose. The ACT was checked as clinically indicated. Arterial and venous sheaths were removed no sooner than 2 hours after discontinuation of argatroban and when the ACT was less than 160 seconds.
If a patient required anticoagulation after the procedure, argatroban could be continued, but at a lower infusion dose between 2.5 and 5 mcg/kg/min. An aPTT was drawn 2 hours after this dose reduction and the dose of argatroban then was adjusted as clinically indicated (not to exceed 10 mcg/kg/min), to reach an aPTT between 1.5 and 3 times baseline value (not to exceed 100 seconds).
In 92 of the 112 interventions (82%), the patient received the initial bolus of 350 mcg/kg and an initial infusion dose of 25 mcg/kg/min. The majority of patients did not require additional bolus dosing during the PCI procedure. The mean value for the initial ACT measurement after the start of dosing for all interventions was 379 sec (median 338 sec; 5th percentile-95th percentile 238 to 675 sec). The mean ACT value per intervention over all measurements taken during the procedure was 416 sec (median 390 sec; 5th percentile-95th percentile 261 to 698 sec). About 65% of patients had ACTs within the recommended range of 300 to 450 seconds throughout the procedure. The investigators did not achieve anticoagulation within the recommended range in about 23% of patients. However, in this small sample, patients with ACTs below 300 seconds did not have more coronary thrombotic events, and patients with ACTs over 450 seconds did not have higher bleeding rates.
Acute procedural success was defined as lack of death, emergent coronary artery bypass graft (CABG), or Q-wave myocardial infarction. Acute procedural success was reported in 98.2% of patients who underwent PCIs with argatroban anticoagulation compared with 94.3% of historical control patients anticoagulated with heparin (p = NS). Among the 112 interventions, 2 patients had emergency CABGs, 3 had repeat PTCAs, 4 had non-Q-wave myocardial infarctions, 3 had myocardial ischemia, 1 had an abrupt closure, and 1 had an impending closure (some patients may have experienced more than 1 event). No patients died. -
16 HOW SUPPLIED/STORAGE AND HANDLING
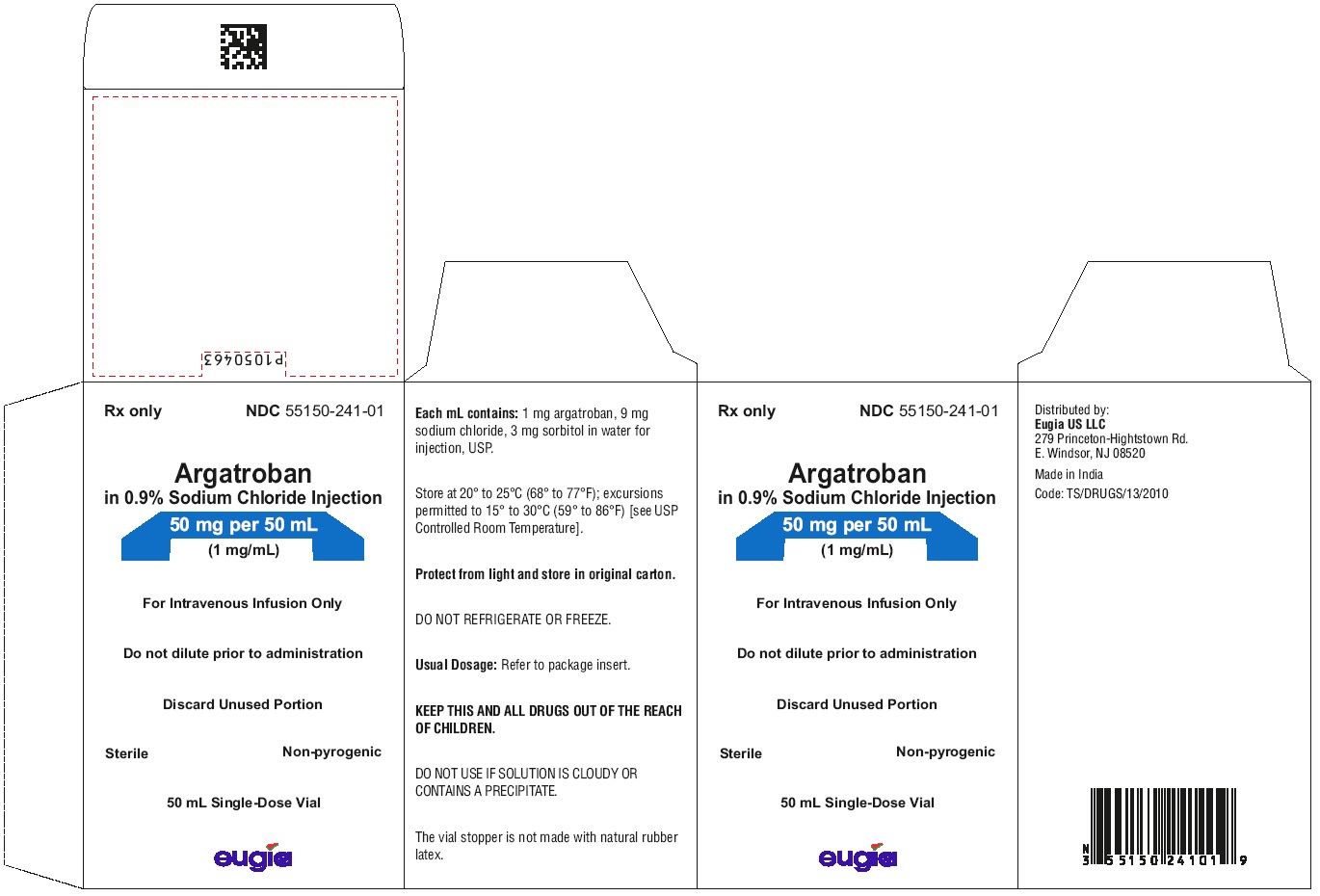
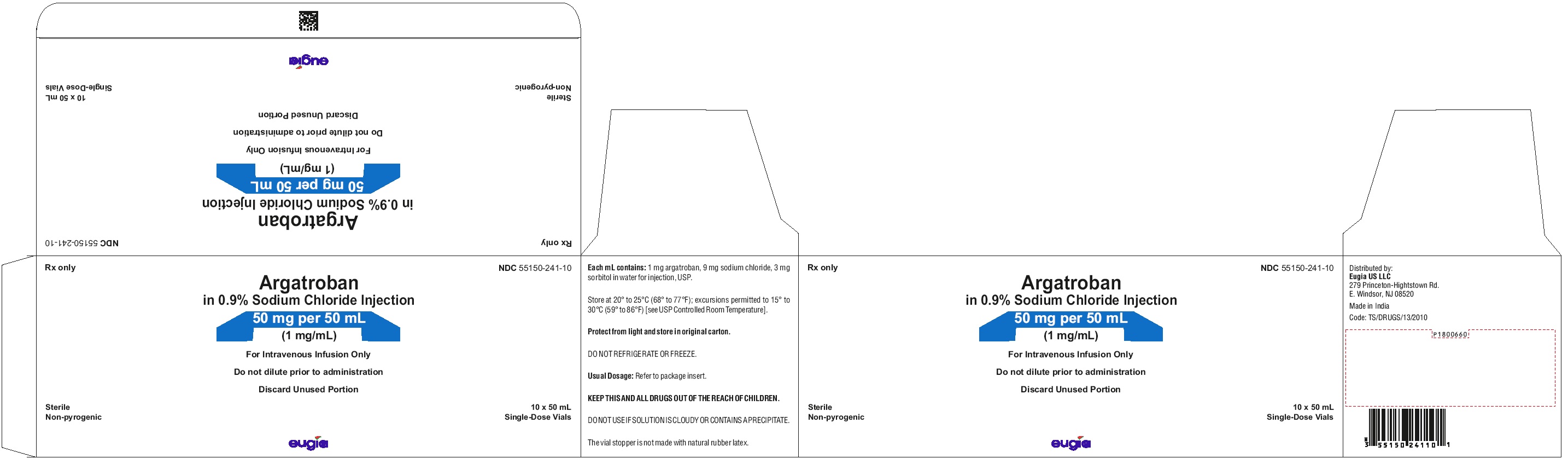
Argatroban in sodium chloride injection is supplied as a single-dose vial containing 50 mg argatroban in 50 mL of aqueous solution (1 mg/mL).
50 mL single-dose vials
packaged in carton of 10 vials NDC 55150-241-10
50 mL single-dose vial
packaged individually NDC 55150-241-01
Storage and Handling:
Store the vials in original cartons at 20º to 25ºC (68º to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Do not refrigerate or freeze. Retain in the original carton to protect from light. If the solution is cloudy, or if an insoluble precipitate is noted, the vial should be discarded.
The vial stopper is not made with natural rubber latex. -
17 PATIENT COUNSELING INFORMATION
Inform patients of the risks associated with Argatroban in sodium chloride injection as well as the plan for regular monitoring during administration of the drug. Specifically, inform patients to report:
- the use of any other products known to affect bleeding [see Warnings and Precautions (5.1)].
- any medical history that may increase the risk for bleeding, including a history of severe hypertension; recent lumbar puncture or spinal anesthesia; major surgery, especially involving the brain, spinal cord, or eye; hematologic conditions associated with increased bleeding tendencies such as congenital or acquired bleeding disorders and gastrointestinal lesions such as ulcerations.
- any bleeding signs or symptoms.
- the occurrence of any signs or symptoms of allergic reactions (e.g., airway reactions, skin reactions and vasodilation reactions) [see Adverse Reactions (6.1)].
Distributed by:
Eugia US LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Eugia Pharma Specialities Limited
Hyderabad - 500032
India - PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg per 50 mL (1 mg / mL) - Container Label
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg per 50 mL (1 mg / mL) - Container-Carton
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 50 mg per 50 mL (1 mg / mL) – Container-Carton (10 Vials)
-
INGREDIENTS AND APPEARANCE
ARGATROBAN
argatroban injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55150-241 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARGATROBAN (UNII: IY90U61Z3S) (ARGATROBAN ANHYDROUS - UNII:OCY3U280Y3) ARGATROBAN ANHYDROUS 50 mg in 50 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55150-241-10 10 in 1 CARTON 11/27/2018 1 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:55150-241-01 1 in 1 CARTON 11/27/2018 2 50 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209552 11/27/2018 Labeler - Eugia US LLC (968961354) Establishment Name Address ID/FEI Business Operations Eugia Pharma Specialities Limited 650498244 ANALYSIS(55150-241) , MANUFACTURE(55150-241) , PACK(55150-241)