Label: CARBIDOPA AND LEVODOPA tablet
- NDC Code(s): 51862-855-01, 51862-855-05, 51862-856-01, 51862-856-05, view more
- Packager: Mayne Pharma Commercial LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 31, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
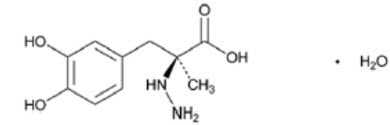
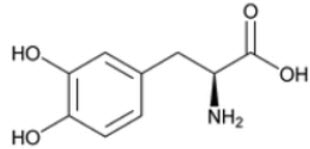
DESCRIPTIONCarbidopa and levodopa tablets are a combination product for the treatment of Parkinson's disease and syndrome. Carbidopa, USP an inhibitor of aromatic amino acid decarboxylation, is a white ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Parkinson's disease is a progressive, neurodegenerative disorder of the extrapyramidal nervous system affecting the mobility and control of the skeletal muscular system. Its ...
-
INDICATIONS AND USAGECarbidopa and levodopa tablets, USP are indicated in the treatment of Parkinson's disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication ...
-
CONTRAINDICATIONSNonselective monoamine oxidase (MAO) inhibitors are contraindicated for use with carbidopa and levodopa tablets. These inhibitors must be discontinued at least two weeks prior to initiating ...
-
WARNINGSWhen carbidopa and levodopa tablets are to be given to patients who are being treated with levodopa, levodopa must be discontinued at least twelve hours before therapy with carbidopa and levodopa ...
-
PRECAUTIONSGeneral - As with levodopa, periodic evaluations of hepatic, hematopoietic, cardiovascular, and renal function are recommended during extended therapy. Patients with chronic wide-angle glaucoma ...
-
ADVERSE REACTIONSThe most common adverse reactions reported with carbidopa and levodopa tablets have included dyskinesias, such as choreiform, dystonic, and other involuntary movements, and nausea. The following ...
-
OVERDOSAGEManagement of acute overdosage with carbidopa and levodopa tablets is the same as management of acute overdosage with levodopa. Pyridoxine is not effective in reversing the actions of carbidopa ...
-
DOSAGE AND ADMINISTRATIONThe optimum daily dosage of carbidopa and levodopa tablets must be determined by careful titration in each patient. Carbidopa and levodopa tablets are available in a 1:4 ratio of carbidopa to ...
-
HOW SUPPLIEDCarbidopa and Levodopa Tablets, USP are supplied as follows: 10 mg/100 mg —Each mottled blue, round flat bevelled tablet, plain on one side, engraved with "m" above the score and "711" below ...
-
SPL UNCLASSIFIED SECTIONMayne Pharma - Greenville, NC 27834 - 61739 - Revised — June 2020 - Rx Only
-
PRINCIPAL DISPLAY PANEL - 10 mg/100 mg Tablet Bottle LabelNDC 51862- 855-01 - Carbidopa - and Levodopa - Tablets, USP - 10mg - /100mg - Rx Only - 100 Tablets - mayne - pharma
-
PRINCIPAL DISPLAY PANEL - 25 mg/100 mg Tablet Bottle LabelNDC 51862- 856-01 - Carbidopa - and Levodopa - Tablets, USP - 25mg - /100mg - Rx Only - 100 Tablets - mayne - pharma
-
PRINCIPAL DISPLAY PANEL - 25 mg/250 mg Tablet Bottle LabelNDC 51862- 858-01 - Carbidopa - and Levodopa - Tablets, USP - 25mg - /250mg - Rx Only - 100 Tablets - mayne - pharma
-
INGREDIENTS AND APPEARANCEProduct Information