Label: DEFEROXAMINE- deferoxamine mesylate injection, powder, lyophilized, for solution
- NDC Code(s): 63323-597-10, 63323-599-30
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 20, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DEFEROXAMINE MESYLATE FOR INJECTION safely and effectively. See full prescribing information for DEFEROXAMINE MESYLATE FOR ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE 1.1 Acute Iron Intoxication - Deferoxamine Mesylate for Injection is indicated as an adjunct to standard measures for the treatment of acute iron intoxication. 1.2 Chronic Iron Overload ...
-
2 DOSAGE AND ADMINISTRATION The dosage (based on body weight in mg/kg/day), rates of administration, and mode of administration for both adults and pediatric patients are individually determined and adapted during the course ...
-
3 DOSAGE FORMS AND STRENGTHS For injection: 500 mg of deferoxamine mesylate (corresponding to 426.82 mg of deferoxamine as free base) is a white to off-white lyophilized powder in a single-dose vial for reconstitution. For ...
-
4 CONTRAINDICATIONS Deferoxamine Mesylate for Injection is contraindicated in patients with: • A history of a hypersensitivity reaction to deferoxamine or any of its inactive ingredients [see Description (11)] ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis, have occurred in Deferoxamine Mesylate for Injection-treated patients. Reactions have included flushing of the ...
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in the labeling: • Hypersensitivity Reactions [see Warnings and Precautions (5.1)] • Auditory and Ocular Toxicity ...
-
7 DRUG INTERACTIONS 7.1 Prochlorperazine - Concurrent treatment with Deferoxamine Mesylate for Injection and prochlorperazine, a phenothiazine derivative, may lead to temporary impairment of consciousness. 7.2 ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - There are no available data on Deferoxamine Mesylate for Injection use in pregnant women to evaluate for a drug-associated risk of major birth defects ...
-
10 OVERDOSAGE Acute Toxicity - Intravenous LD50s (mg/kg): mice, 287; rats, 329. Inadvertent administration of an overdose or inadvertent intravenous bolus administration/rapid intravenous infusion may be ...
-
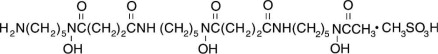
11 DESCRIPTION Deferoxamine Mesylate for Injection, USP is an iron-chelating agent, available in vials for injection via intramuscular, subcutaneous, and intravenous administration. Deferoxamine Mesylate for ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Deferoxamine Mesylate for Injection chelates iron by forming a stable complex that prevents the iron from entering into further chemical reactions. It readily chelates ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies in animals have not been performed with Deferoxamine Mesylate for Injection. Cytotoxicity may occur ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING How Supplied - Deferoxamine Mesylate for Injection, USP is supplied in single-dose vials containing 500 mg and 2 g of deferoxamine mesylate (corresponding to 426.82 mg and 1.707 g of ...
-
17 PATIENT COUNSELING INFORMATION Caution patients about the potential allergic reactions associated with rapid intravenous administration of Deferoxamine Mesylate for Injection and the need for monitoring allergic reactions ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY - Deferoxamine Mesylate 500 mg per vial Vial Label - NDC 63323-597-10 509710 - Deferoxamine Mesylate for Injection, USP - 500 mg per vial - For subcutaneous ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY - Deferoxamine Mesylate 500 mg per vial Carton Panel - NDC 63323-597-10 509710 - Deferoxamine Mesylate for Injection, USP - 500 mg per vial - For ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY - Deferoxamine Mesylate 2 grams per vial Vial Label - NDC 63323-599-30 509930 - Deferoxamine Mesylate for Injection, USP - 2 grams per vial - For ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL - PRINCIPAL DISPLAY - Deferoxamine Mesylate 2 grams per vial Carton Panel - NDC 63323-599-30 509930 - Deferoxamine Mesylate for Injection, USP - 2 grams per vial - For ...
-
INGREDIENTS AND APPEARANCEProduct Information