Imiquimod Cream
-
Important: For use on the skin only (topical).Do not use Imiquimod Cream in or near your lips, mouth, eyes or inside your anus, vagina, or nose.
What is Imiquimod ...
Imiquimod Cream
Important: For use on the skin only (topical).Do not use Imiquimod Cream in or near your lips, mouth, eyes or inside your anus, vagina, or nose.
What is Imiquimod Cream?
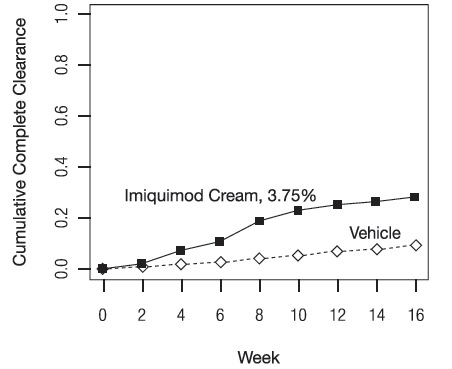
- Imiquimod Cream, 3.75% is a prescription medicine used on the skin (topical) to treat actinic keratosis on the face or balding scalp in adults with a normal immune system.
- Imiquimod Cream, 3.75% is a prescription medicine used on the skin (topical) to treat warts on the outside of the genitals (external) and around the outside of the anus (perianal) in people 12 years and older.
It is not known if Imiquimod Cream is safe and effective in the treatment of:
- human papilloma virus (HPV) disease of the urethra, the inside of the vagina (intravaginal), cervix, rectum, or inside of the anus (intra-anal)
- actinic keratosis, when treated with more than one 2-cycle treatment course in the same affected area
- people with extreme sensitivity to sunlight (xeroderma pigmentosum)
- a type of skin cancer called superficial basal cell carcinoma
- people who have a weakened immune system
It is not known if Imiquimod Cream is safe and effective for the treatment of actinic keratosis in children less than 18 years of age.
It is not known if Imiquimod Cream is safe and effective in children less than 12 years of age for external genital and perianal warts.
Before using Imiquimod Cream, tell your healthcare provider about all of your medical conditions, including if you:
- have problems with your immune system, including autoimmune disease or long-lasting (chronic) graft versus host disease
- are being treated or have been treated with other medicines or surgery. You should not use Imiquimod Cream until your skin has healed from other treatments.
- have other skin problems or sunburn.
- are pregnant or plan to become pregnant. It is not known if Imiquimod Cream can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Imiquimod Cream passes into your breast milk. If you breastfeed during treatment with Imiquimod Cream, avoid contact of your treated skin with your baby’s mouth or eyes. Talk to your healthcare provider about the best way to feed your baby if you use Imiquimod Cream.
Tell your healthcare provider about all the medicines you take,including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Imiquimod Cream?
- Use Imiquimod
Cream exactly as your healthcare provider tells you to use it.
- Your healthcare provider should show you how to apply Imiquimod Cream.
- Apply Imiquimod
Cream 1 time a day just before your bedtime. Do not use more Imiquimod Cream than you need to cover the affected area. Using too much Imiquimod Cream, or using it too often, or for too long can increase your chances for having a severe skin reaction or other side effects.
- Your healthcare provider may prescribe Imiquimod Cream in a packet or pump.
-
Imiquimod Cream packet:
- Open a packet of Imiquimod Cream just before use.
- After applying Imiquimod Cream, the opened packet should be thrown away even if all the Imiquimod Cream was not used.
-
Imiquimod Cream pump:
- Remove the cap.
- Before using the pump
for the first time only, prime the pump by pressing the top of the pump all the way down repeatedly until the cream appears. The cream obtained from priming should be dispensed into a tissue and then thrown away (discarded). The pump is now primed and is ready to use. You will not have to repeat this priming process during your treatment.
- When dispensing the cream, slightly tilt the pump as shown.
-
- Firmly press the top of the pump all the way down to dispense the cream onto your hand or fingertip.
Applying Imiquimod Cream:
- Wash the area where Imiquimod Cream will be applied with mild soap and water.
- Allow the area to dry.
- Wash your hands well.
- Apply a thin layer of Imiquimod Cream
onlyto the affected area(s) to be treated.
- Rub Imiquimod Cream in all the way until you cannot see the cream on the affected area(s).
- Wash your hands well after applying Imiquimod Cream.
- After about 8 hours, wash the treated area(s) with mild soap and water.
Do notleave Imiquimod Cream on your skin longer than prescribed. If you forget to apply Imiquimod Cream, apply the next dose of Imiquimod Cream at your regularly scheduled time.
When using Imiquimod Cream for actinic keratosis:
-
Do not get Imiquimod Cream in or near your lips, mouth, or eyes, or inside your nose.
- If you get Imiquimod Cream in your mouth or in your eyes, rinse well with water right away.
- Apply Imiquimod Cream to the skin of the affected area for 2 weeks, then stop using for 2 weeks, then apply again for 2 weeks.
- If you have been prescribed Imiquimod Cream packets, do not use more than 2 packets for each daily application.
- If you have been prescribed Imiquimod Cream pump, do not dispense more than 2 pumps for each daily application.
When using Imiquimod Cream for external genital or perianal warts:
-
Do not get Imiquimod Cream in your anus or vagina.
- Treatment should continue until the warts are completely gone or for up to 8 weeks. Imiquimod Cream will not cure your genital or perianal warts. New warts may develop during treatment with Imiquimod Cream.
- Uncircumcised males treating warts under their penis foreskin must pull their foreskin back and clean before treatment and clean daily during treatment.
- Females treating genital warts must be careful when applying Imiquimod Cream around the vaginal opening.
- If you have been prescribed Imiquimod Cream packets, do not use more than 1 packet for each daily application.
- If you have been prescribed Imiquimod Cream pump, do not dispense more than 1 pump for each daily application.
What should I avoid while using Imiquimod Cream?
-
Do notcover the treated area with bandages or other closed dressings. Cotton gauze dressings can be used, if needed. Cotton underwear can be worn after applying Imiquimod Cream to the genital or perianal area.
-
Do notuse sunlamps or tanning beds, and avoid sunlight as much as possible during treatment with Imiquimod Cream. Use sunscreen and wear protective clothing if you go outside during daylight.
-
Do nothave sexual contact including genital, anal, or oral sex when Imiquimod Cream is on your genital or perianal skin. Imiquimod Cream may weaken condoms and vaginal diaphragms. This means they may not work as well to prevent pregnancy.
- Avoid using Imiquimod Cream and any other Imiquimod products in the same treatment area. They have the same active ingredients (Imiquimod) and may increase your risk for side effects.
What are the possible side effects of Imiquimod Cream?
ImiquimodCream may cause serious side effects,including:
-
-
Treatment site skin reactions.Skin reactions including drainage (weeping) or breakdown of the outer layer of your skin (erosion) can happen after a few applications of Imiquimod Cream. Swelling outside of the vagina (vulvar swelling) may happen in females. Females should take special care if applying the cream at the opening of the vagina because skin reactions can cause pain or swelling, and may cause problems passing urine. If you get skin reactions, wash the treated area with mild soap and water.
-
Stop Imiquimod Cream right away and call your healthcare provider if you get any skin reactions that affect your daily activities, or that do not go away.
-
Flu-like symptoms.Tell your healthcare provider if you get tiredness, nausea, vomiting, fever, chills, muscle pain, and joint pain.
Your healthcare provider may temporarily stop or completely stop your treatment with Imiquimod Cream if you develop
treatment site skin reactions or flu-like symptoms.
The most common side effects of Imiquimod Cream include:
-
- skin reactions including skin redness, scabbing, crusting, flaking, scaling, dryness, swelling
- headache
- itching at the treatment area
- tiredness
- nausea
- skin irritation
- pain at the treatment area
These are not all of the possible side effects of Imiquimod Cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store Imiquimod Cream?
-
- Store Imiquimod Cream at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Imiquimod Cream pumps upright.
- Do not freeze.
- Safely throw away Imiquimod Cream that is out of date, unused or partially used.
Keep Imiquimod Cream and all medicines out of the reach of children.
General information about the safe and effective use of Imiquimod Cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Imiquimod Cream for a condition for which it was not prescribed. Do not give Imiquimod Cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Imiquimod Cream that is written for health professionals.
What are the ingredients in Imiquimod Cream?
Active Ingredient:Imiquimod
Inactive Ingredients:benzyl alcohol, cetyl alcohol, glycerin, isostearic acid, methylparaben, polysorbate 60, propylparaben, purified water, sorbitan monostearate, stearyl alcohol, white petrolatum, and xanthan gum.
Distributed by:
Oceanside Pharmaceuticals, a division of Bausch Health US, LLC, Bridgewater, NJ 08807 USA
Manufactured by:
Bausch Health Companies Inc., Laval, Quebec H7L 4A8, Canada
Patented. See https://patents.ortho-dermatologics.com for US patent information.
© 2024 Bausch Health Companies Inc. or its affiliates
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 09/2024
9740901
Close