Label: OPTASE- glycerin solution/ drops
- NDC Code(s): 72972-004-01
- Packager: Scope Health Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
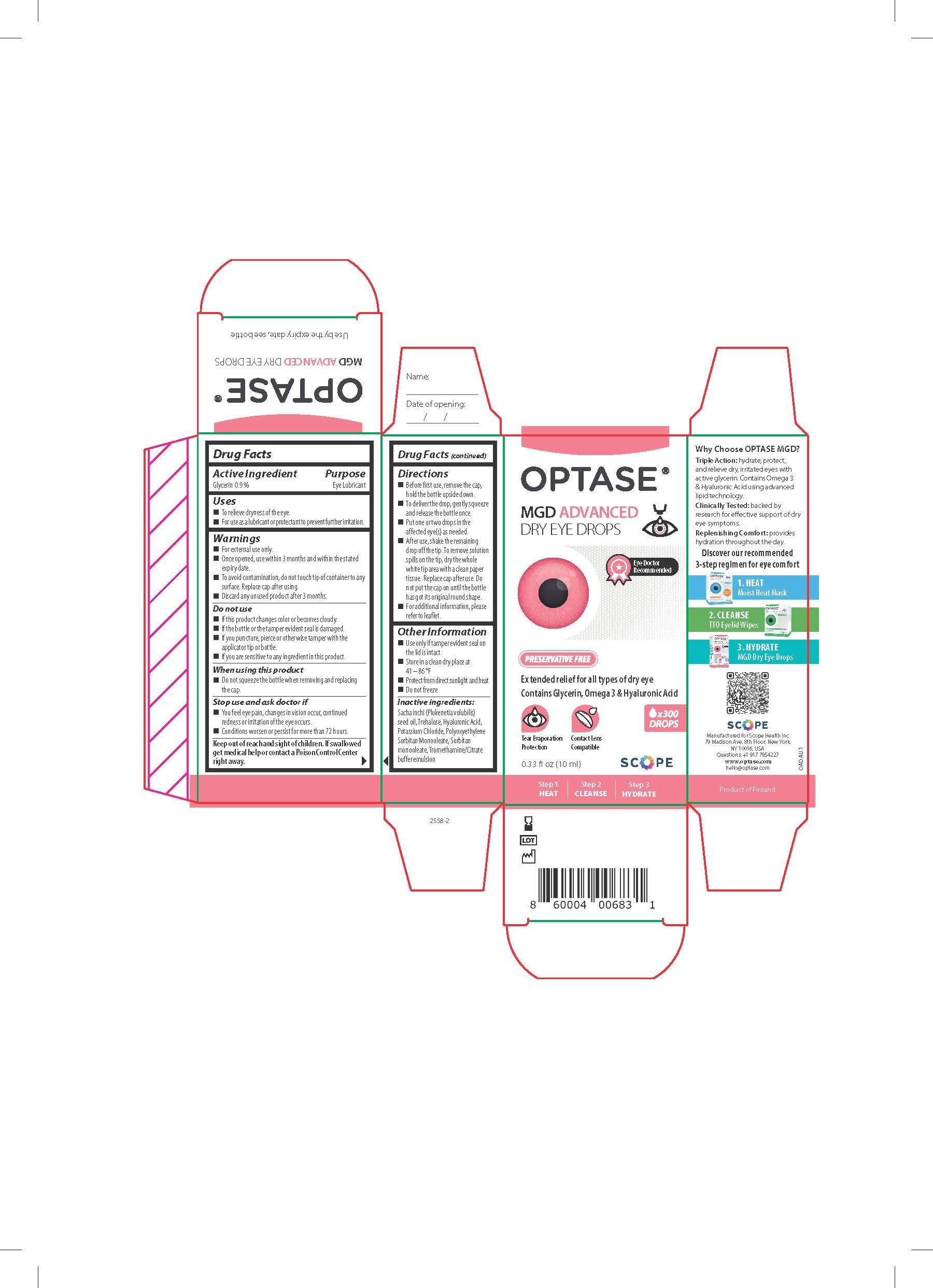
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
WARNINGS
Warning
• For external use only.
• To avoid contamination, do not touch tip
of container to any surface. After use, shake the bottle
downwards in order to remove any residual drop that
may be left. Replace cap after using.
• Do not use the solution if it changes color or becomes
cloudy
Stop use and ask doctor if
• You feel eye pain.
• Changes in vision occur.
• Redness or irritation of the eye lasts.
• Condition worsens or lasts more than 72 hours. - INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- OTHER SAFETY INFORMATION
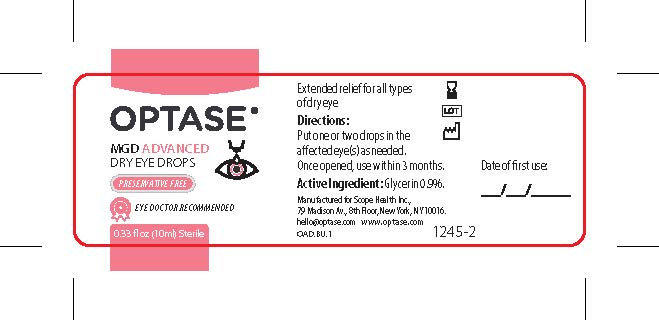
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OPTASE
glycerin solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72972-004 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) TREHALOSE (UNII: B8WCK70T7I) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) TROMETHAMINE (UNII: 023C2WHX2V) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) POTASSIUM CHLORIDE (UNII: 660YQ98I10) PLUKENETIA VOLUBILIS SEED OIL (UNII: 8ED72Z8J1Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72972-004-01 1 in 1 BOX 02/22/2022 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/22/2022 Labeler - Scope Health Inc (116778693) Registrant - Oy Finnsusp Ab (369374137)