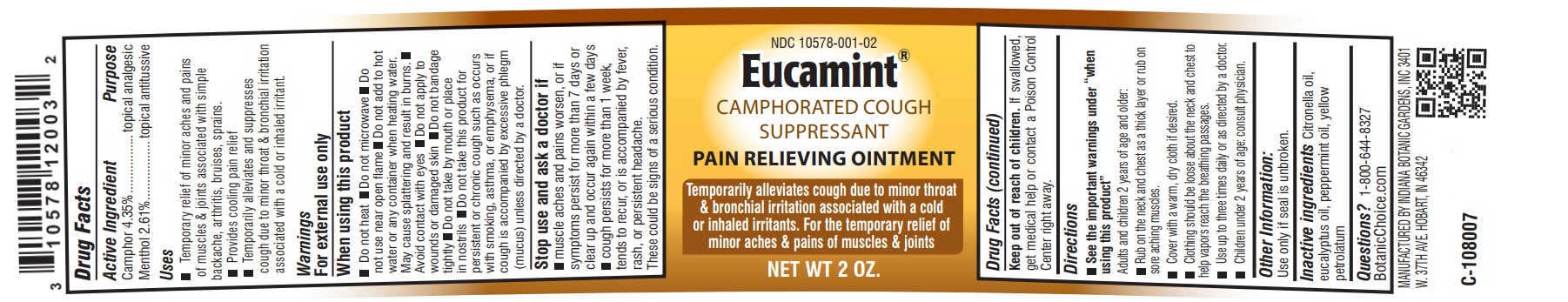
Label: EUCAMINT- menthol, camphor ointment
- NDC Code(s): 10578-001-02, 10578-001-04
- Packager: Indiana Botanic Gardens
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings:
-
When using this product
Do not heat
Do not microwave
Do not add to hot water or any container when heating water. May cause splattering and result in burns.
Avoid contact with eyes
Do not apply to wounds or damaged skin
Do not bandage tightly
Do not take by mouth or place in nostrils
Do not take or give this product for persistent or chronic cough such as occurs with asthma or if cough is accompanied by excessive phlegm (mucous) unless directed by a doctor.
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
See the important warnings under "when using this product"
Adults and children 2 years of age and older:
Rub on the neck and chest as a thick layer or rub on sore aching muscles.
Cover with a warm, dry cloth if desired.
Clothing should be loose about the neck and chest to help vapors reach the breathing passages.
Use up to three times daily or as directed by a doctor.
Children under 2 years of age: consult physician.
- Other Information:
- Inactive Ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EUCAMINT
menthol, camphor ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10578-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2.61 g in 100 g CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4.35 g in 100 g Inactive Ingredients Ingredient Name Strength PETROLATUM (UNII: 4T6H12BN9U) EUCALYPTUS OIL (UNII: 2R04ONI662) PEPPERMINT OIL (UNII: AV092KU4JH) CITRONELLA OIL (UNII: QYO8Q067D0) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10578-001-02 57 g in 1 JAR; Type 0: Not a Combination Product 01/01/1995 2 NDC:10578-001-04 114 g in 1 JAR; Type 0: Not a Combination Product 01/01/1995 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/01/1995 Labeler - Indiana Botanic Gardens (005421771) Establishment Name Address ID/FEI Business Operations Indiana Botanic Gardens 005421771 manufacture(10578-001)