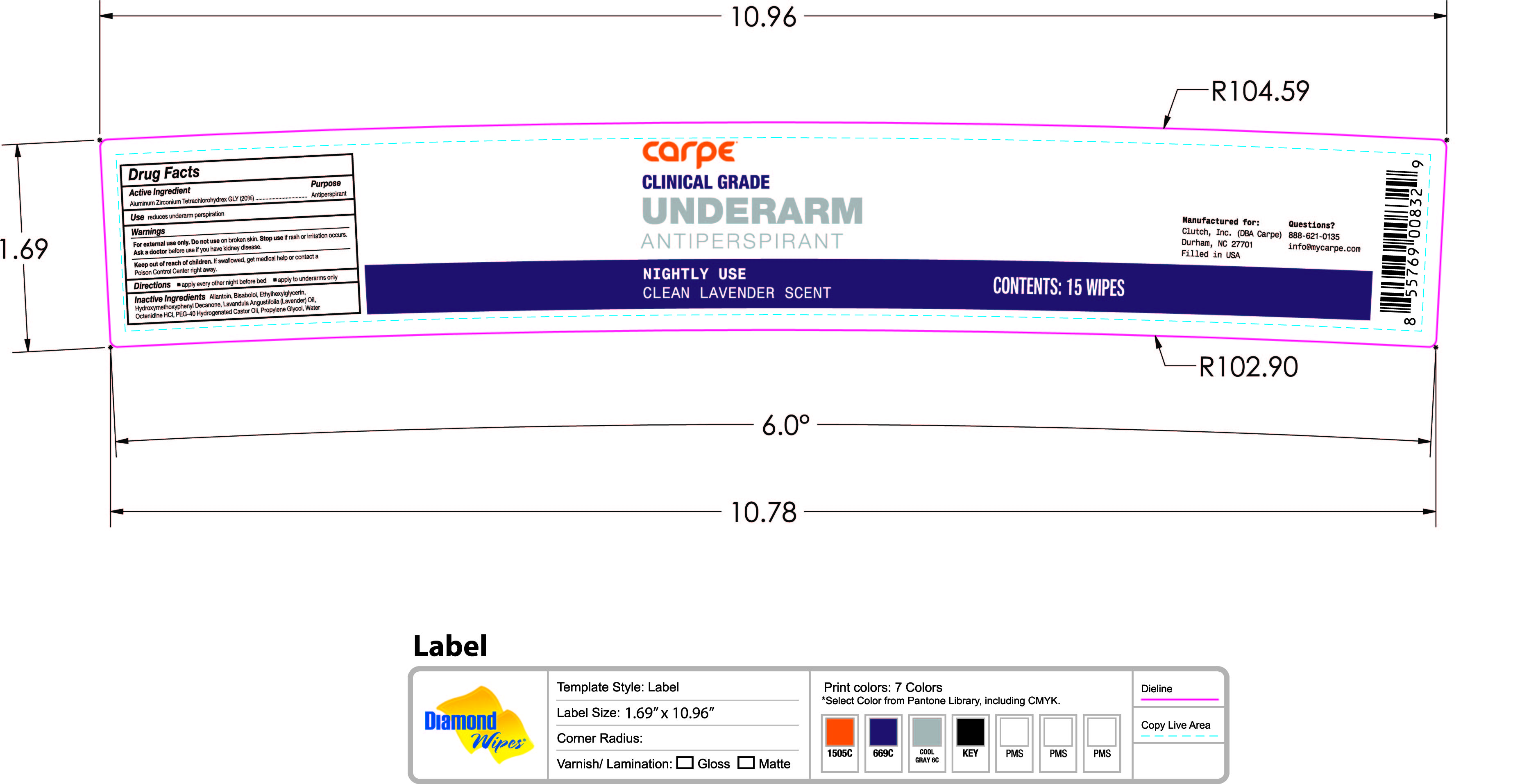
Label: UNDERARM ANTIPERSPIRANT PM WIPES CLEAN LAVENDER SCENT- aluminum zirconium tetrachlorohydrex gly solution
- NDC Code(s): 74307-010-01, 74307-010-02
- Packager: Clutch Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDERARM ANTIPERSPIRANT PM WIPES CLEAN LAVENDER SCENT
aluminum zirconium tetrachlorohydrex gly solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74307-010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY (UNII: 8O386558JE) (ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY - UNII:8O386558JE) ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 20 g in 100 g Inactive Ingredients Ingredient Name Strength LAVENDER OIL (UNII: ZBP1YXW0H8) OCTENIDINE HYDROCHLORIDE (UNII: U84956NU4B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) ALLANTOIN (UNII: 344S277G0Z) LEVOMENOL (UNII: 24WE03BX2T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) 1-(4-HYDROXY-3-METHOXYPHENYL)-DECAN-3-ONE (UNII: BO24ID7E9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74307-010-02 15 in 1 JAR 03/15/2021 1 NDC:74307-010-01 106 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 03/15/2021 Labeler - Clutch Inc (080214892)