Label: PHENTERMINE HYDROCHLORIDE capsule
- NDC Code(s): 68788-7972-1, 68788-7972-3
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 11534-176
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Phentermine Hydrochloride Capsules USP safely and effectively. See full prescribing information for Phentermine Hydrochloride ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE Phentermine hydrochloride capsules are indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the ...
-
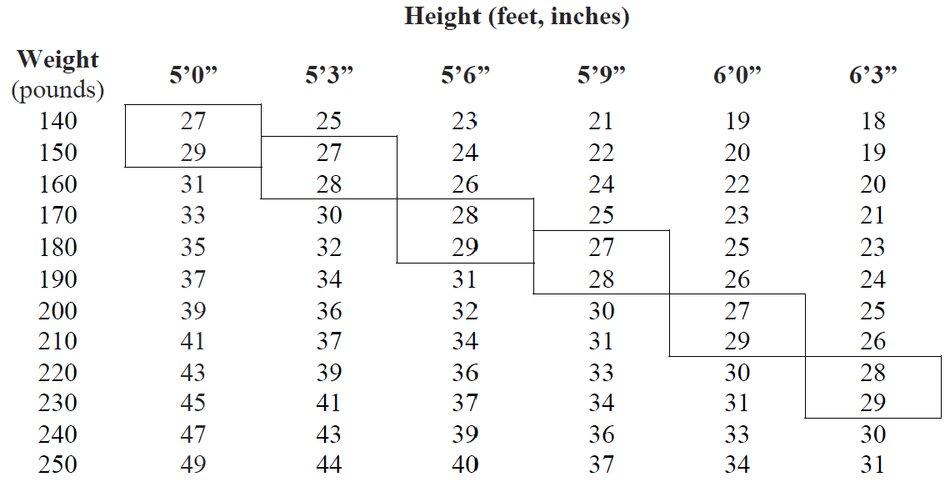
2 DOSAGE AND ADMINISTRATION 2.1 Exogenous Obesity - Dosage should be individualized to obtain an adequate response with the lowest effective dose. The usual adult dose is 15 mg to 30 mg as prescribed by the physician, at ...
-
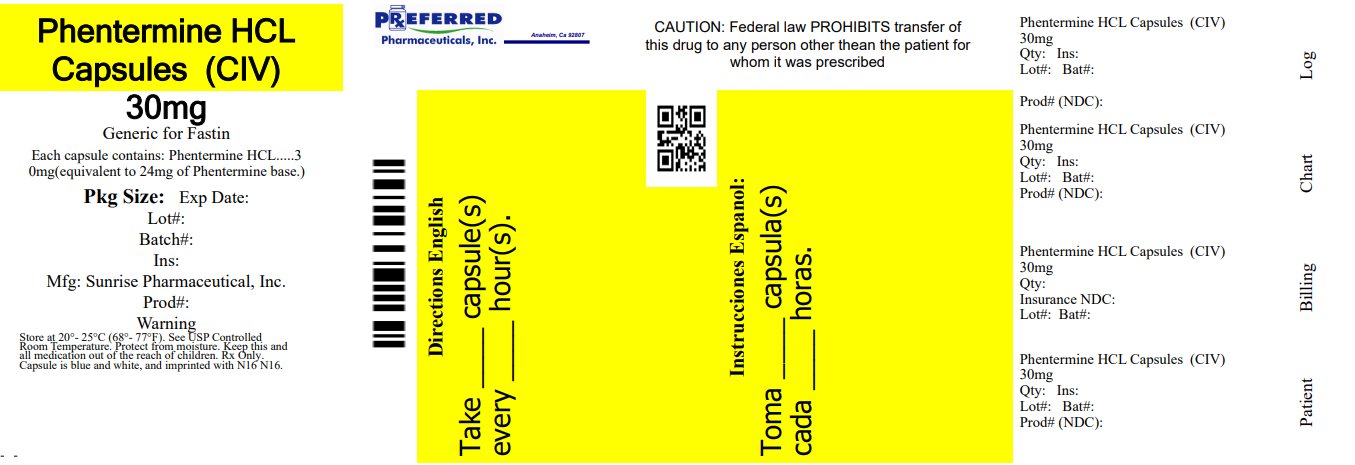
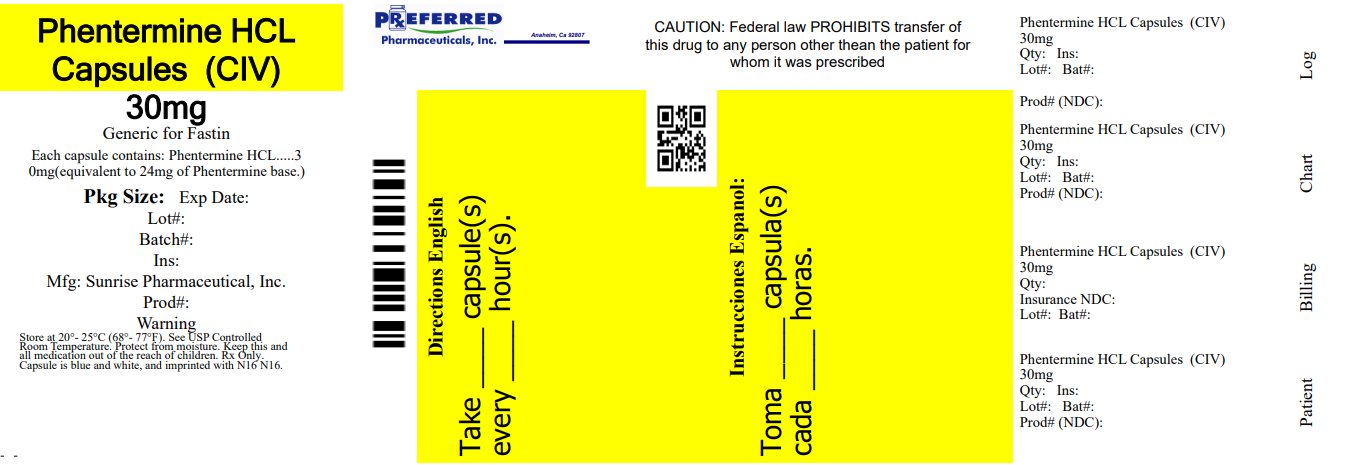
3 DOSAGE FORMS AND STRENGTHS Capsules containing 15 mg or 30 mg phentermine hydrochloride (equivalent to 12 mg or 24 mg phentermine base, respectively). 15 mg capsules: gray opaque cap, yellow opaque body with black imprint ...
-
4 CONTRAINDICATIONS • History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension) • During or within 14 days following the ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Co-administration with Other Drug Products for Weight Loss - Phentermine hydrochloride capsules are indicated only as short-term (a few weeks) monotherapy for the management of exogenous ...
-
6 ADVERSE REACTIONS The following adverse reactions are described, or described in greater detail, in other sections: • Primary pulmonary hypertension [see WARNINGS AND PRECAUTIONS (5.2)] • Valvular heart disease ...
-
7 DRUG INTERACTIONS 7.1 Monoamine Oxidase Inhibitors - Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy Pregnancy categoryX - Phentermine is contraindicated during pregnancy because weight loss offers no potential benefit to a pregnant woman and may result in fetal harm. A ...
-
9 DRUG ABUSE AND DEPENDENCE 9.1 Controlled Substance Phentermine is a Schedule IV controlled substance. 9.2 Abuse Phentermine is related chemically and pharmacologically to the amphetamines. Amphetamines and ...
-
10 OVERDOSAGE The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage. 10.1 Acute Overdosage - Manifestations of acute overdosage include ...
-
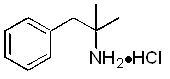
11 DESCRIPTION Phentermine hydrochloride is a sympathomimetic amine anorectic. Its chemical name is α,α, dimethylphenethylamine hydrochloride. The structural formula is as ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action Phentermine is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, amphetamine (d- and ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility Studies have not been performed with phentermine to determine the potential for carcinogenesis, mutagenesis or impairment of ...
-
14 CLINICAL STUDIES In relatively short-term clinical trials, adult obese subjects instructed in dietary management and treated with “anorectic” drugs lost more weight on the average than those treated with placebo ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING Phentermine hydrochloride capsules USP, for oral administration, are available as: 30 mg: Blue/Clear powder filled capsules; imprinted “N16” in black ink and supplied as: NDC 68788-7972-1 ...
-
17 PATIENT COUNSELING INFORMATION Patients must be informed that phentermine hydrochloride is a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL NDC 68788-7972 - Phentermine Hydrochloride Capsules USP CIV - 30 mg - Blue/Clear - Rx Only - SUNRISE PHARMA - Repackaged By: Preferred Pharmaceuticals Inc.
-
INGREDIENTS AND APPEARANCEProduct Information