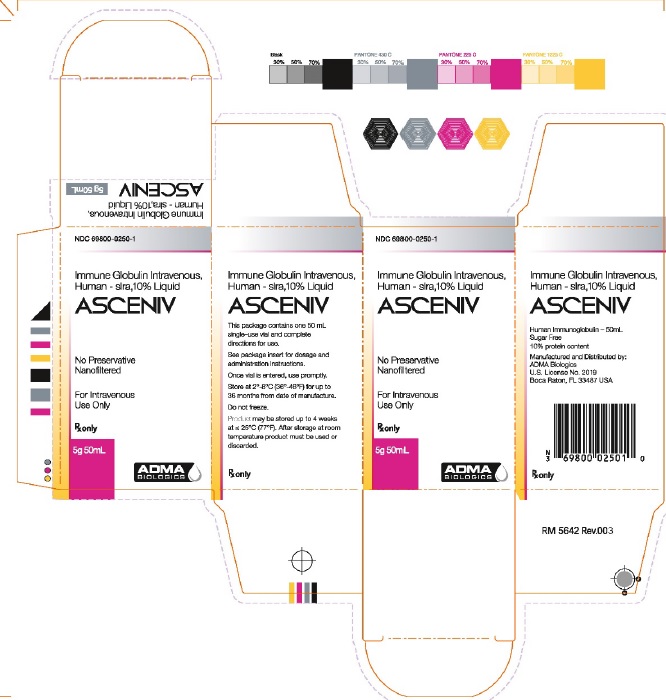
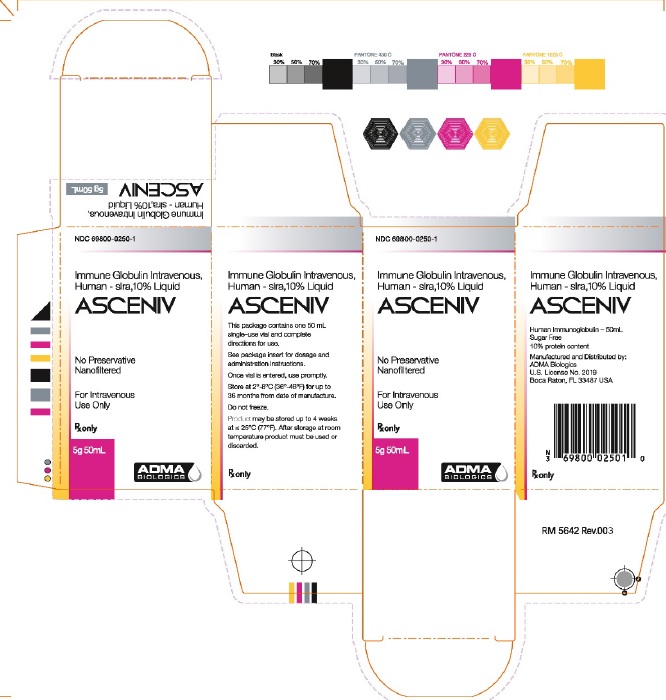
Label: ASCENIV- human immunoglobulin g liquid
- NDC Code(s): 69800-0250-1, 69800-0250-2
- Packager: ADMA Biologics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 25, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ASCENIV - ™ safely and effectively. See full prescribing information for ASCENIV. ASCENIV (immune globulin ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: THROMBOSIS, RENAL DYSFUNCTION AND ACUTE RENAL FAILURE
- Thrombosis may occur with immune globulin (IGIV) products, including ASCENIV. Risk factors may include: advanced age, prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors (see Warnings and Precautions [5.2], Patient Counseling Information [17]).
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with the administration of IGIV products in predisposed patients.
- Renal dysfunction and acute renal failure occur more commonly in patients receiving IGIV products containing sucrose. ASCENIV does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction or renal failure, administer ASCENIV at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity ( see Dosage and Administration [2.1, 2.3], Warnings and Precautions [5.3]).
-
1 INDICATIONS AND USAGEASCENIV (immune globulin intravenous, human – slra) is a 10% immune globulin liquid for intravenous injection, indicated for the - treatment of primary humoral immunodeficiency (PI) in adults ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dose - The recommended dose of ASCENIV for replacement therapy in primary humoral immunodeficiency (PI) is 300 to 800 mg/kg body - weight administered every 3 to 4 weeks. The dose may ...
-
3 DOSAGE FORMS AND STRENGTHSASCENIV is a liquid solution containing 10% IgG (100 mg/mL) for intravenous infusion.
-
4 CONTRAINDICATIONSASCENIV is contraindicated in: patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. IgA-deficiency patients with antibodies to IgA and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Severe hypersensitivity reactions may occur with IGIV products, including ASCENIV. In case of hypersensitivity, discontinue ASCENIV infusion immediately and institute ...
-
6 ADVERSE REACTIONSThe most common adverse reactions to ASCENIV (reported in ≥5% of clinical study subjects) were headache, sinusitis, diarrhea, gastroenteritis viral, nasopharyngitis, upper respiratory tract ...
-
7 DRUG INTERACTIONSImmunoglobulin administration may transiently impair the efficacy of live attenuated virus vaccines such as measles, mumps, rubella, and varicella because the continued presence of high levels of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - No human data are available to indicate the presence or absence of drug-associated risk. Animal reproduction studies have not been conducted with ASCENIV. It is ...
-
10 OVERDOSAGEWith intravenous administration, overdose may lead to fluid overload and hyperviscosity. Patients at risk of complications of fluid overload and hyperviscosity include elderly patients and those ...
-
11 DESCRIPTIONASCENIV is a purified, sterile, ready-to-use preparation of concentrated human immunoglobulin G (IgG) antibodies. The product is a clear to opalescent liquid, which is colorless to pale yellow ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - ASCENIV is a replacement therapy for patients with primary humoral immunodeficiency (PI) (e.g. agammaglobulinemia, hypogammaglobulinemia, CVID, SCID). The broad ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No animal studies were conducted to evaluate the carcinogenic or mutagenic effects of ASCENIV or its effects on fertility. 13.2 ...
-
14 CLINICAL STUDIESA prospective, open-label, single-arm, multicenter trial assessed the efficacy, safety, and pharmacokinetics of ASCENIV in adult and pediatric subjects with PI. Study subjects were receiving ...
-
15 REFERENCESGupta N, Ahmed I, Nissel-Horowitz S, Patel D, Mehrotra B. Intravenous gammaglobulin-associated acute renal failure. Am J Hematol 2001; 66:151-152. Cayco, A.V., M.A. Perazella, and J.P. Hayslett ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGASCENIV is supplied in a single-use, tamper-evident vial. The components used in the packaging for ASCENIV are not made with natural rubber latex. ASCENIV is supplied in 50 mL size containing 5 ...
-
17 PATIENT COUNSELING INFORMATIONInstruct patients taking ASCENIV to immediately report symptoms of: Thrombosis which includes pain and/or swelling of an arm or legs/feet with warmth over the - affected area ...
-
NDC: 69800-0250-2 - Vial Label

-
NDC: 69800-0250-1 - Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information