Label: MEPROBAMATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 55111-640-01, 55111-640-10, 55111-641-01, 55111-641-05 - Packager: Dr.Reddy's Laboratories Limited
- This is a repackaged label.
- Source NDC Code(s): 67787-261, 67787-262
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CIV
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 9, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
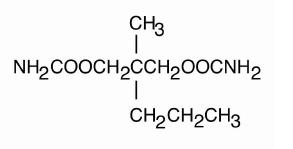
DESCRIPTIONMeprobamate is awhite powder with a characteristic odor and a bittertaste. It is slightly soluble in water, freely soluble in acetone andalcohol, and sparingly soluble in ether. The structural ...
-
CLINICAL PHARMACOLOGYMeprobamate is a carbamate derivative which has been shown inanimal studies to have effects at multiple sites in the central nervoussystem including the thalamus and limbic system.
-
INDICATIONS AND USAGEMeprobamate tablets are indicated for the management of anxietydisorders or for the short-term relief of the symptoms of anxiety.Anxiety or tension associated with the stress of everyday life ...
-
CONTRAINDICATIONSAcute intermittent porphyria as well as allergic or idiosyncraticreactions to meprobamate or related compounds such ascarisoprodol, mebutamate, tybamate, or carbromal.
-
WARNINGSDrug Dependence - Physical dependence, psychological dependence, and abuse haveoccurred. When chronic intoxication from prolonged use occurs, itusually involves ingestion of greater than ...
-
PRECAUTIONSThe lowest effective dose should be administered, particularly toelderly and/or debilitated patients, in order to preclude oversedation. The possibility of suicide attempts should be considered ...
-
ADVERSE REACTIONSCentral Nervous System - Drowsiness, ataxia, dizziness, slurred speech, headache, vertigo,weakness, paresthesias, impairment of visual accommodation,euphoria, overstimulation, paradoxical ...
-
OVERDOSAGESuicidal attempts with meprobamate have resulted in drowsiness,lethargy, stupor, ataxia, coma, shock, vasomotor, and respiratorycollapse. Some suicidal attempts have been fatal. The following data ...
-
DOSAGE AND ADMINISTRATIONMeprobamate Tablets USP: The usual adult daily dosage is 1200 mgto 1600 mg, in three or four divided doses; adaily dosage above 2400mg is not recommended. The usual daily dosage for children ages ...
-
HOW SUPPLIEDMeprobamate Tablets USP 200 mg are white, round, biconvex tabletsdebossed with I and 7 on one side and bisect on the other. Supplied in bottles of 100 and 1000. Bottles of 100: NDC ...
-
SPL UNCLASSIFIED SECTION200 mg - CONTAINER LABELS - 100's count - 1000's count - 400 mg - CONTAINER LABELS - 100's count - 500's count
-
INGREDIENTS AND APPEARANCEProduct Information