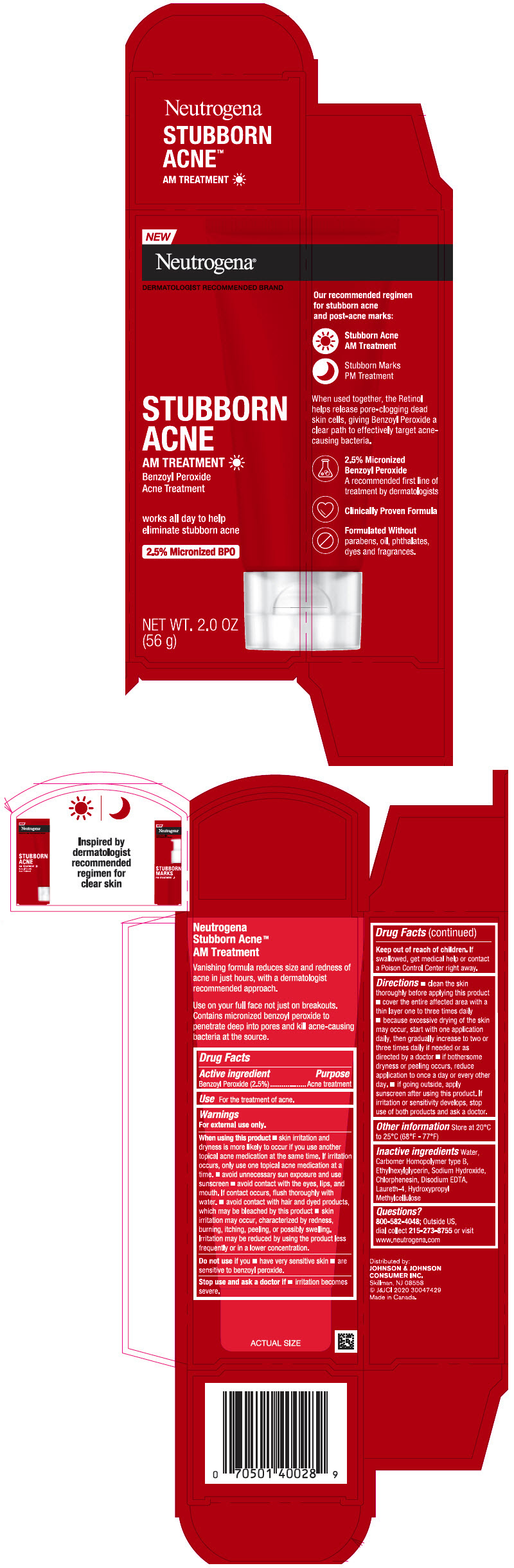
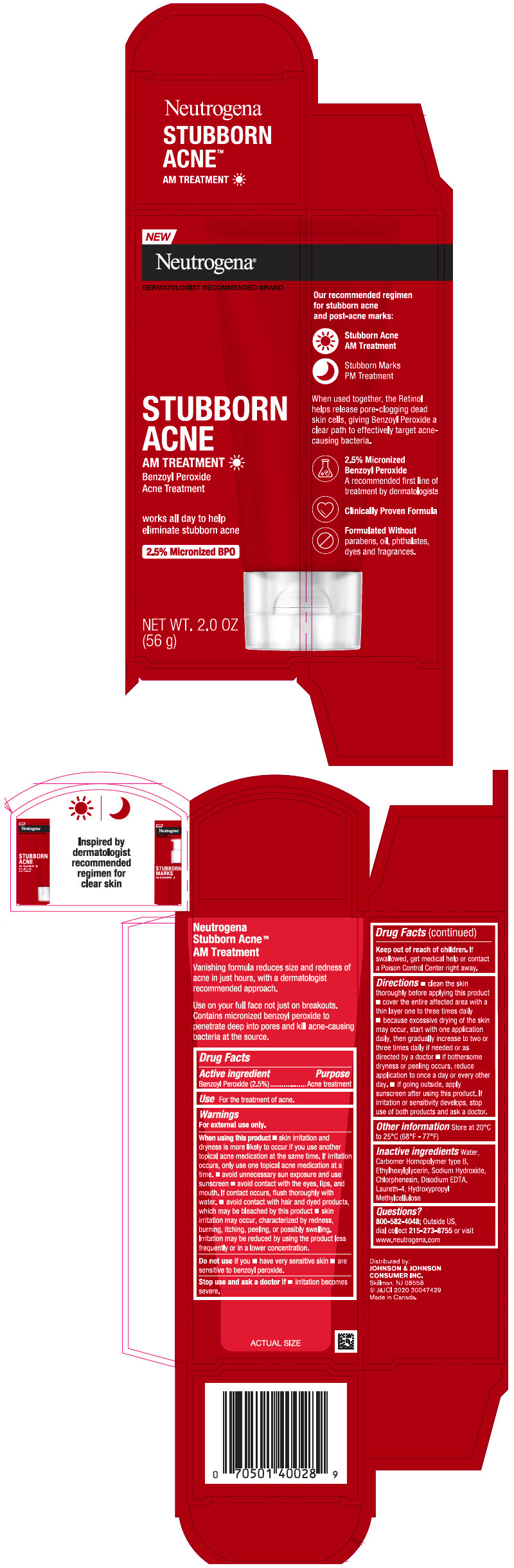
Label: NEUTROGENA STUBBORN ACNE AM TREATMENT- benzoyl peroxide gel
- NDC Code(s): 69968-0653-1, 69968-0653-2
- Packager: Kenvue Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time.
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth. If contact occurs, flush thoroughly with water
- avoid contact with hair or dyed products, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- If going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other Information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 56 g Tube Carton
-
INGREDIENTS AND APPEARANCE
NEUTROGENA STUBBORN ACNE AM TREATMENT
benzoyl peroxide gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0653 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 25 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM HYDROXIDE (UNII: 55X04QC32I) CHLORPHENESIN (UNII: I670DAL4SZ) EDETATE DISODIUM (UNII: 7FLD91C86K) LAURETH-4 (UNII: 6HQ855798J) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0653-2 1 in 1 CARTON 08/03/2020 1 56 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69968-0653-1 12 in 1 PACKAGE 08/03/2020 2 9 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/03/2020 Labeler - Kenvue Brands LLC (118772437)