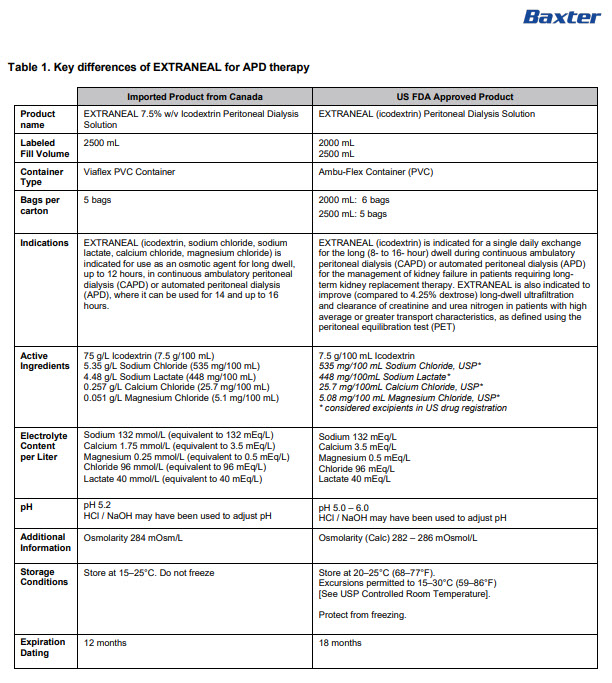
Label: EXTRANEAL- icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solution
- NDC Code(s): 0941-0709-01, 0941-0709-05
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
JB9923L 2500 mL DIN 02240806
2D barcode
(01)00085412150116Extraneal 7.5% Icodextrin Peritoneal
Dialysis SolutionFOR INTRAPERITONEAL ADMINISTRATION
APPROX 90 mL EXCESS / EXCES APPROX DE 90 mL
NONPYROGENIC / STERILE / APYROGENE
APPROX pH 5.2PER/PAR 1000 mL
ICODEXTRIN / ICODEXTRINE – 75 g
SODIUM CHLORIDE / CHLORURE DE SODIUM USP – 5.35 g
SODIUM LACTATE / LACTATE DE SODIUM – 4.48 g
CALCIUM CHLORIDE DIHYDRATE / DIHYRATE DE CHLORURE DE CALCIUM USP -
257 mg
MAGNESIUM CHLORIDE HEXAHYDRATE / HEXAHYDRATE DE CHLORURE DE
MAGNESIUM USP – 51 mg
IN WATER FOR INJECTIONS / DANS DE L’EAU POUR INJECTION USP
THEORETICAL OSMOLARITY 284 mOsmol/L /
OSMOLARITE THEORIQUE 284 mOsmol/LAPPROX mmol/L Na – 132 Ca – 1.75 Mg – 0.25 Cl – 96 LACTATE – 40
NOT TO BE USED FOR INTRAVENOUS INFUSION
FOR DOSAGE ADMINISTRATION AND DETAILED DIRECTIONS FOR USE SEE
PACKAGE INSERT / PRODUCT MONOGRAPH AVAILABLE UPON REQUEST /
DO NOT USE UNLESS SOLUTION IS CLEAR AND CONTAINER IS UNDAMAGED
/ DISCARD UNUSED PORTION / STORE AT 15°C TO 25°C / DO NOT FREEZE /
KEEP OUT OF REACH OF CHILDRENExtraneal Solution d’Icodextrine à 7,5 % pour
Dialyse PéritonéalePOUR ADMINISTRATION PAR VOIE INTRAPERITONEALE
NE PAS ADMINSTRER PAR PERFUSION INTRAEINEUSE / CONSULTER LA
NOTICE D’EMBALLAGE POUR CONNAITRE LES RENSEIGNEMENTS SUR LA
POSOLOGIE L’ADMINISTRATION ET LE MODE D’EMPLOI MONOGRAPHIE DU
PRODUIT DISPONIBLE SUR DEMANDE / NE PAS EMPLOYER SI LA SOLUTION
EST TROUBLE OU SI LE SAC EST ENDOMMAGE / JETER TOUTE PORTION
INUTILISEE / GARDER ENTRE 15 °C / PROTEGER DU GEL / GARDER
HORS DE LA PORTEE DES ENFANTSPVC CONTAINER / CONTENANT DE PVC
BAXTER AND EXTRANEAL ARE TRADEMARKS OF BAXTER INTERNATIONAL INC
BAXTER ET EXTRANEAL SONT DES MARQUES DE COMMERCE DE BAXTER INTERNATIONAL INCBaxterLogo
Baxter Corporation
Mississauga ON L5N 0C2Non Latex Symbol
88-70-20-372500 500
250 250
Store at/Garder entre 15° C to/et 25° C
5-2500 mL IN/DANS 3000 mLJB-99-23LP ICODEXTRIN 7.5% ICODEXTRINE
EXTRANEAL™ PD SOLUTION
SOLUTION POR DPLOT: WWWWWWWWW EXP: 2099 99
2d Barcode
(01)50085412150111 -
INGREDIENTS AND APPEARANCE
EXTRANEAL
icodextrin, sodium chloride, sodium lactate, calcium chloride, magnesium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0709 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ICODEXTRIN (UNII: 2NX48Z0A9G) (ICODEXTRIN - UNII:2NX48Z0A9G) ICODEXTRIN 75 g in 1000 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 5.35 g in 1000 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 4.48 g in 1000 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 257 mg in 1000 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 51 mg in 1000 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0709-05 5 in 1 CARTON 11/10/2024 1 NDC:0941-0709-01 2500 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/10/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Corporation 205087968 analysis(0941-0709) , label(0941-0709) , manufacture(0941-0709) , sterilize(0941-0709) , pack(0941-0709)