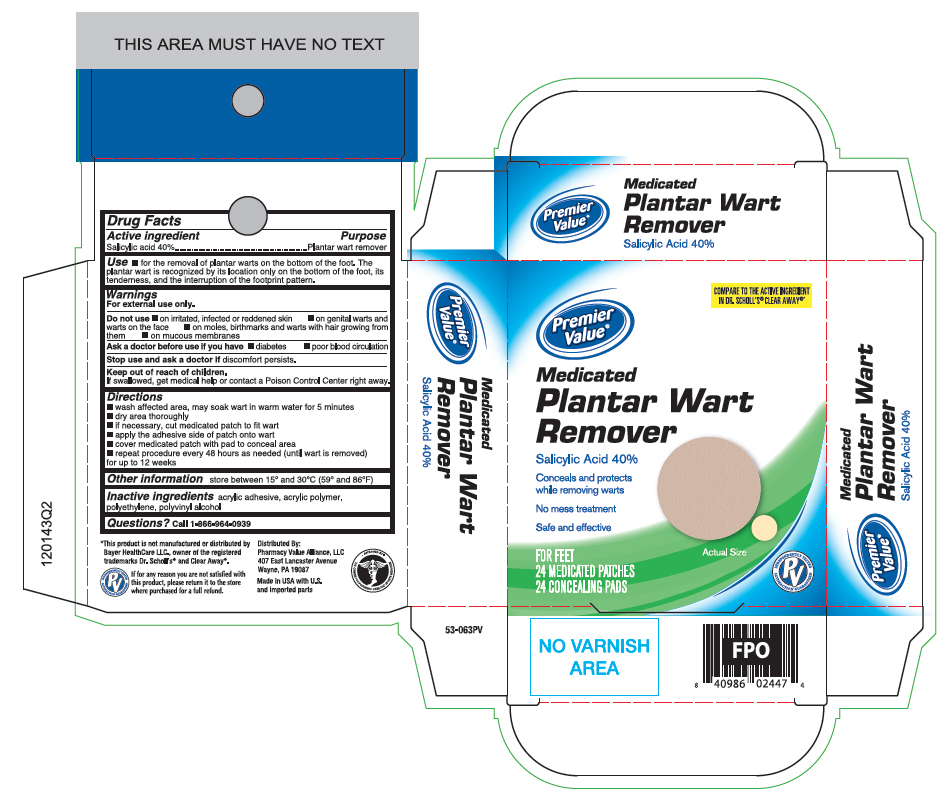
Label: SALICYLIC ACID- plantar wart remover patch
- NDC Code(s): 68016-618-00
- Packager: Chain Drug Consortium, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Do not use
- if you are diabetic or have poor blood circulation, except under the advise and supervision of a doctor or podiatrist
- on irritated, infected or reddened skin
- on genital warts and warts on the face
- on moles, birthmarks and warts with hair growing from them
- on mucous membranes
- Directions
- Other information
- Inactive ingredients
- Questions?
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SALICYLIC ACID
plantar wart remover patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-618 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 mg in 24 Inactive Ingredients Ingredient Name Strength POLYVINYL ALCOHOL (UNII: 532B59J990) VINYL ACETATE (UNII: L9MK238N77) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-618-00 24 in 1 PACKAGE; Type 0: Not a Combination Product 02/19/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M028 02/19/2009 Labeler - Chain Drug Consortium, LLC (101668460)