Label: ONDANSETRON injection, solution
- NDC Code(s): 0409-0009-02, 0409-0009-25
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 6, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ONDANSETRON safely and effectively. See full prescribing information for ONDANSETRON. ONDANSETRON injection, for intravenous or ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Prevention of Nausea and Vomiting Associated With Initial and Repeat Courses of Emetogenic Cancer Chemotherapy - Ondansetron Injection is indicated for the prevention of nausea and vomiting ...
-
2 DOSAGE AND ADMINISTRATION2.1 Prevention of Nausea and Vomiting Associated With Initial and Repeat Courses of Emetogenic Chemotherapy - Important Preparation Instructions - • Dilution of Ondansetron Injection in 50 mL ...
-
3 DOSAGE FORMS AND STRENGTHSOndansetron Injection, USP, 2 mg/mL is a clear, colorless, nonpyrogenic, sterile solution available as a 2 mL single-dose vial.
-
4 CONTRAINDICATIONSOndansetron Injection is contraindicated for patients known to have hypersensitivity (e.g., anaphylaxis) to this product or any of its components. Anaphylactic reactions have been reported in ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity Reactions - Hypersensitivity reactions, including anaphylaxis and bronchospasm, have been reported in patients who have exhibited hypersensitivity to other selective 5-HT3 ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: • Hypersensitivity Reactions [see Warnings and Precautions (5.1)] • QT Prolongation [see Warnings ...
-
7 DRUG INTERACTIONS7.1 Drugs Affecting Cytochrome P-450 Enzymes - Ondansetron does not appear to induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system of the liver. Because ondansetron is ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Published epidemiological studies on the association between ondansetron use and major birth defects have reported inconsistent findings and have important ...
-
9 DRUG ABUSE AND DEPENDENCEAnimal studies have shown that ondansetron is not discriminated as a benzodiazepine nor does it substitute for benzodiazepines in direct addiction studies.
-
10 OVERDOSAGEThere is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual intravenous doses as large as 150 mg and total daily intravenous ...
-
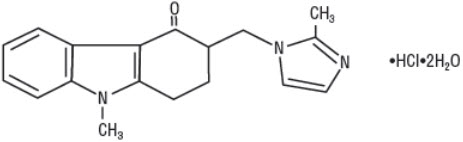
11 DESCRIPTIONThe active ingredient of Ondansetron Injection, USP is ondansetron hydrochloride, a selective blocking agent of the serotonin 5-HT3 receptor type. Its chemical name is (±) 1, 2, 3, 9 ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Ondansetron is a selective 5-HT3 receptor antagonist. While ondansetron's mechanism of action has not been fully characterized, it is not a dopamine-receptor ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 and 30 mg/kg per day ...
-
14 CLINICAL STUDIESThe clinical efficacy of ondansetron hydrochloride, the active ingredient of ondansetron, was assessed in clinical trials as described below. 14.1 Chemotherapy-Induced Nausea and ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGOndansetron Injection, USP, 2 mg/mL, is supplied as follows: Unit of SaleConcentration - NDC 0409-0009-25 - Tray of 25 - 2 mL Single-dose Vials - 4 mg/2 mL - (2 mg/mL) Storage: Store at ...
-
17 PATIENT COUNSELING INFORMATIONHypersensitivity Reactions - Inform patients that Ondansetron Injection may cause hypersensitivity reactions, some as severe as anaphylaxis and bronchospasm. The patient should report any signs ...
-
SPL UNCLASSIFIED SECTIONDistributed by Hospira, Inc., Lake Forest, IL 60045 USA - PREMIERProRx® is a registered trademark of Premier Healthcare Alliance, L.P., used under license. LAB-1600-1.0
-
PRINCIPAL DISPLAY PANEL - 2 mL Vial Label2 mL Single-dose Vial - NDC 0409-0009-02 - Rx only - Ondansetron - Injection, USP - 4 mg/2 mL (2 mg/mL) PROTECT FROM LIGHT - Dist. by Hospira, Inc., Lake Forest, IL 60045 USA
-
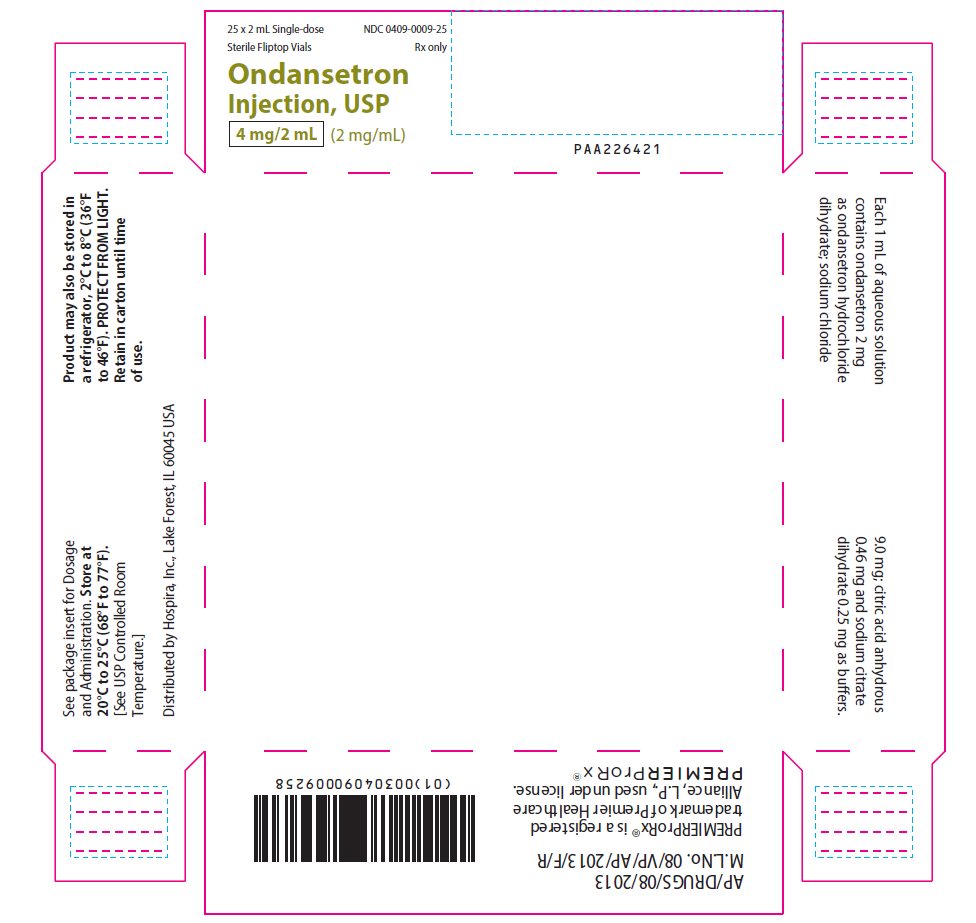
PRINCIPAL DISPLAY PANEL - 2 mL Vial Carton25 x 2 mL Single-dose - Sterile Fliptop Vials - NDC 0409-0009-25 - Rx only - Ondansetron - Injection, USP - 4 mg/2 mL (2 mg/mL) PAA226421
-
INGREDIENTS AND APPEARANCEProduct Information