Label: LABETALOL HYDROCHLORIDE tablet, film coated
-
NDC Code(s):
51407-614-01,
51407-614-05,
51407-615-01,
51407-615-05, view more51407-616-01
- Packager: Golden State Medical Supply, Inc.
- This is a repackaged label.
- Source NDC Code(s): 70377-060, 70377-061, 70377-062
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Labetalol Hydrochloride Tablets, USP are adrenergic receptor blocking agents that have both selective alpha 1-adrenergic and non-selective
beta-adrenergic receptor blocking actions in a single substance.Labetalol hydrochloride (HCl), USP is a racemate, chemically designated as 2-hydroxy-5-[1-hydroxy-2-[(1-methyl-3-phenylpropyl) amino]
ethyl] benzamide monohydrochloride and it has the following structural formula: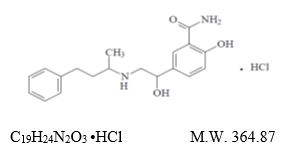
Labetalol HCl, USP has two asymmetric centers and therefore exists as a molecular complex of two diastereoisomeric pairs. Dilevalol, the R,R' stereoisomer, makes up 25% of racemic labetalol.
Labetalol HCl, USP is a white to off-white crystalline powder, soluble in water.
Each tablet, for oral administration, contains 100 mg, 200 mg, or 300 mg of labetalol hydrochloride, USP. In addition, each tablet contains the following inactive ingredients: corn starch, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, polysorbate 80, sodium starch glycolate and titanium dioxide.
-
CLINICAL PHARMACOLOGY
Labetalol HCl combines both selective, competitive alpha 1-adrenergic blocking and nonselective, competitive beta-adrenergic blocking activity in a single substance. In man, the ratios of alpha-to beta-blockade have been estimated to be approximately 1:3 and 1:7 following oral and intravenous administration, respectively. Beta 2- agonist activity has been demonstrated in animals with minimal beta 1-agonist (ISA) activity detected. In animals, at doses greater than those required for alpha- or beta-adrenergic blockade, a membrane-stabilizing effect has been demonstrated.
Pharmacodynamics
The capacity of labetalol HCl to block alpha receptors in man has been demonstrated by attenuation of the pressor effect of phenylephrine and by a significant reduction of the pressor response caused by immersing the hand in ice-cold water (“cold-pressor test”). Labetalol HCl's beta 1-receptor blockade in man was demonstrated by a small decrease in the resting heart rate, attenuation of tachycardia produced by isoproterenol or exercise, and by attenuation of the reflex tachycardia to the hypotension produced by amyl nitrite. Beta 2-receptor blockade was demonstrated by inhibition of the isoproterenol-induced fall in diastolic blood pressure. Both the alpha- and beta-blocking actions of orally administered labetalol HCl contribute to a decrease in blood pressure in hypertensive patients. Labetalol HCl consistently, in dose-related fashion, blunted increases in exercise-induced blood pressure and heart rate, and in their double product. The pulmonary circulation during exercise was not affected by labetalol HCl dosing.
Single oral doses of labetalol HCl administered to patients with coronary artery disease had no significant effect on sinus rate, intraventricular conduction, or QRS duration. The atrioventricular (A-V) conduction time was modestly prolonged in two of seven patients. In another study, intravenous labetalol HCl slightly prolonged A-V nodal conduction time and atrial effective refractory period with only small changes in heart rate. The effects on A-V nodal refractoriness were inconsistent.
Labetalol HCl produces dose-related falls in blood pressure without reflex tachycardia and without significant reduction in heart rate, presumably through a mixture of its alpha- and beta-blocking effects. Hemodynamic effects are variable, with small nonsignificant changes in cardiac output seen in some studies but not others, and small decreases in total peripheral resistance. Elevated plasma renins are reduced.
Doses of labetalol HCl that controlled hypertension did not affect renal function in mild to severely hypertensive patients with normal
renal function.Due to the alpha 1-receptor blocking activity of labetalol HCl, blood pressure is lowered more in the standing than in the supine position, and symptoms of postural hypotension (2%), including rare instances of syncope, can occur. Following oral administration, when postural hypotension has occurred, it has been transient and is uncommon when the recommended starting dose and titration increments are closely followed (see DOSAGE AND ADMINISTRATION) . Symptomatic postural hypotension is most likely to occur 2 to 4 hours after a dose, especially following the use of large initial doses or upon large changes in dose.
The peak effects of single oral doses of labetalol HCl occur within 2 to 4 hours. The duration of effect depends upon dose, lasting at least 8 hours following single oral doses of 100 mg and more than 12 hours following single oral doses of 300 mg. The maximum, steady-state blood pressure response upon oral, twice-a-day dosing occurs within 24 to 72 hours.
The antihypertensive effect of labetalol has a linear correlation with the logarithm of labetalol plasma concentration, and there is also a linear correlation between the reduction in exercise-induced tachycardia occurring at 2 hours after oral administration of labetalol HCl and the logarithm of the plasma concentration.
About 70% of the maximum beta-blocking effect is present for 5 hours after the administration of a single oral dose of 400 mg, with suggestion that about 40% remains at 8 hours.
The antianginal efficacy of labetalol HCl has not been studied. In 37 patients with hypertension and coronary artery disease, labetalol HCl did not increase the incidence or severity of angina attacks.
Exacerbation of angina and, in some cases, myocardial infarction and ventricular dysrhythmias have been reported after abrupt discontinuation of therapy with beta-adrenergic blocking agents in patients with coronary artery disease. Abrupt withdrawal of these agents in patients without coronary artery disease has resulted in transient symptoms, including tremulousness, sweating, palpitation, headache, and malaise. Several mechanisms have been proposed to explain these phenomena, among them increased sensitivity to catecholamines because of increased numbers of beta receptors.
Although beta-adrenergic receptor blockade is useful in the treatment of angina and hypertension, there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. Beta-adrenergic blockade may worsen A-V block by preventing the necessary facilitating effects of sympathetic activity on conduction. Beta 2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm, and it may also interfere with exogenous bronchodilators in such patients.
Pharmacokinetics and Metabolism
Labetalol HCl is completely absorbed from the gastrointestinal tract with peak plasma levels occurring 1 to 2 hours after oral administration. The relative bioavailability of labetalol HCl tablets compared to an oral solution is 100%. The absolute bioavailability (fraction of drug reaching systemic circulation) of labetalol when compared to an intravenous infusion is 25%; this is due to extensive “first-pass” metabolism. Despite “first-pass” metabolism, there is a linear relationship between oral doses of 100 to 3,000 mg and peak plasma levels. The absolute bioavailability of labetalol is increased when administered with food.
The plasma half-life of labetalol following oral administration is about 6 to 8 hours. Steady-state plasma levels of labetalol during repetitive dosing are reached by about the third day of dosing. In patients with decreased hepatic or renal function, the elimination half-life of labetalol is not altered; however, the relative bioavailability in hepatically impaired patients is increased due to decreased “first-pass” metabolism.
The metabolism of labetalol is mainly through conjugation to glucuronide metabolites. These metabolites are present in plasma and are excreted in the urine and, via the bile, into the feces. Approximately 55% to 60% of a dose appears in the urine as conjugates or unchanged labetalol HCl within the first 24 hours of dosing.
Labetalol has been shown to cross the placental barrier in humans. Only negligible amounts of the drug crossed the blood-brain barrier in animal studies. Labetalol is approximately 50% protein bound. Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol from the general circulation (<1%).
Elderly Patients
Some pharmacokinetic studies indicate that the elimination of labetalol is reduced in elderly patients. Therefore, although elderly patients may initiate therapy at the currently recommended dosage of 100 mg b.i.d., elderly patients will generally require lower maintenance dosages than non-elderly patients. - INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Labetalol HCl tablets are contraindicated in bronchial asthma, overt cardiac failure, greater-than-first degree heart block, cardiogenic shock, severe bradycardia, other conditions associated with severe and prolonged hypotension, and in patients with a history of hypersensitivity to any component of the product (see WARNINGS) .
Beta-blockers, even those with apparent cardioselectivity, should not be used in patients with a history of obstructive airway disease, including asthma.
-
WARNINGS
Hepatic Injury
Severe hepatocellular injury, confirmed by rechallenge in at least one case, occurs rarely with labetalol therapy. The hepatic injury is usually reversible, but hepatic necrosis and death have been reported. Injury has occurred after both short- and long-term treatment and may be slowly progressive despite minimal symptomatology. Similar hepatic events have been reported with a related research compound, dilevalol HCl, including two deaths. Dilevalol HCl is one of the four isomers of labetalol HCl. Thus, for patients taking labetalol, periodic determination of suitable hepatic laboratory tests would be appropriate. Appropriate laboratory testing should be done at the first symptom/sign of liver dysfunction (e.g., pruritus, dark urine, persistent anorexia, jaundice, right upper quadrant tenderness, or unexplained "flu-like" symptoms). If the patient has laboratory evidence of liver injury or jaundice, labetalol should be stopped and not restarted.
Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure. Beta-blockade carries a potential hazard of further depressing myocardial contractility and precipitating more severe failure. Although beta-blockers should be avoided in overt congestive heart failure, if necessary, labetalol HCl can be used with caution in patients with a history of heart failure who are well compensated. Congestive heart failure has been observed in patients receiving labetalol HCl. Labetalol HCl does not abolish the inotropic action of digitalis on heart muscle.
In Patients Without a History of Cardiac Failure
In patients with latent cardiac insufficiency, continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or be given a diuretic, and the response should be observed closely. If cardiac failure continues despite adequate digitalization and diuretic, therapy with labetalol HCl should be withdrawn (gradually, if possible).
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Angina pectoris has not been reported upon labetalol HCl discontinuation. However, hypersensitivity to catecholamines has been observed in patients withdrawn from beta-blocker therapy; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing chronically administered labetalol HCl tablets, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, therapy with labetalol tablets should be reinstituted promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue therapy with labetalol HCl tablets abruptly in patients being treated for hypertension.
Nonallergic Bronchospasm (e.g., Chronic Bronchitis and Emphysema)
Patients with bronchospastic disease should, in general, not receive beta-blockers. Labetalol may be used with caution, however, in patients who do not respond to, or cannot tolerate, other antihypertensive agents. It is prudent, if labetalol HCl tablets are used, to use the smallest effective dose, so that inhibition of endogenous or exogenous beta-agonists is minimized.
Pheochromocytoma
Labetalol HCl has been shown to be effective in lowering blood pressure and relieving symptoms in patients with pheochromocytoma. However, paradoxical hypertensive responses have been reported in a few patients with this tumor; therefore, use caution when administering labetalol HCl to patients with pheochromocytoma.
Diabetes Mellitus and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of premonitory signs and symptoms (e.g., tachycardia) of acute hypoglycemia. This is especially important with labile diabetics. Beta-blockade also reduces the release of insulin in response to hyperglycemia; it may therefore be necessary to adjust the dose of antidiabetic drugs.
Major Surgery
Do not routinely withdraw chronic beta blocker therapy to surgery. The effect of labetalol's alpha-adrenergic activity has not been evaluated in this setting.
A synergism between labetalol HCl and halothane anesthesia has been shown (see PRECAUTIONS-Drug Interactions).
-
PRECAUTIONS
General
Impaired Hepatic Function
Labetalol HCl tablets should be used with caution in patients with impaired hepatic function since metabolism of the drug may be diminished.
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients treated with alpha 1-blockers (labetalol is an alpha/beta blocker). This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs ,and potential prolapse of the iris toward the phacoemulsification incisions. The patient’s ophthalmologist should be prepared for possible modifications to the surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha 1-blocker therapy prior to cataract surgery.
Jaundice or Hepatic Dysfunction
(see WARNINGS).
Information for Patients
As with all drugs with beta-blocking activity, certain advice to patients being treated with labetalol HCl is warranted. This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects. While no incident of the abrupt withdrawal phenomenon (exacerbation of angina pectoris) has been reported with labetalol HCl, dosing with labetalol HCl tablets should not be interrupted or discontinued without a physician's advice. Patients being treated with labetalol HCl tablets should consult a physician at any signs or symptoms of impending cardiac failure or hepatic dysfunction (see WARNINGS). Also, transient scalp tingling may occur, usually when treatment with labetalol HCl tablets is initiated (see ADVERSE REACTIONS).
Laboratory Tests
As with any new drug given over prolonged periods, laboratory parameters should be observed over regular intervals. In patients with concomitant illnesses, such as impaired renal function, appropriate tests should be done to monitor these conditions.
Drug Interactions
In one survey, 2.3% of patients taking labetalol HCl in combination with tricyclic antidepressants experienced tremor as compared to 0.7% reported to occur with labetalol HCl alone. The contribution of each of the treatments to this adverse reaction is unknown but the possibility of a drug interaction cannot be excluded.
Drugs possessing beta-blocking properties can blunt the bronchodilator effect of beta-receptor agonist drugs in patients with bronchospasm; therefore, doses greater than the normal antiasthmatic dose of beta-agonist bronchodilator drugs may be required.
Cimetidine has been shown to increase the bioavailability of labetalol HCl. Since this could be explained either by enhanced absorption or by an alteration of hepatic metabolism of labetalol HCl, special care should be used in establishing the dose required for blood pressure control in such patients.
Synergism has been shown between halothane anesthesia and intravenously administered labetalol HCl. During controlled hypotensive anesthesia using labetalol HCl in association with halothane, high concentrations (3% or above) of halothane should not be used because the degree of hypotension will be increased and because of the possibility of a large reduction in cardiac output and an increase in central venous pressure. The anesthesiologist should be informed when a patient is receiving labetalol HCl.
Labetalol HCl blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effect. If labetalol HCl is used with nitroglycerin in patients with angina pectoris, additional antihypertensive effects may occur.
Care should be taken if labetalol is used concomitantly with calcium antagonists of the verapamil type.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Drug/Laboratory Test Interactions
The presence of labetalol metabolites in the urine may result in falsely elevated levels of urinary catecholamines, metanephrine, normetanephrine, and vanillylmandelic acid when measured by fluorimetric or photometric methods. In screening patients suspected of having a pheochromocytoma and being treated with labetalol HCl, a specific method, such as a high performance liquid chromatographic assay with solid phase extraction (e.g., J Chromatogr 385:241,1987) should be employed in determining levels of catecholamines.
Labetalol HCl has also been reported to produce a false-positive test for amphetamine when screening urine for the presence of drugs using the commercially available assay methods TOXI-LAB ® A (thinlayer chromatographic assay) and EMIT-d.a.u. ® (radioenzymatic assay). When patients being treated with labetalol have a positive urine test for amphetamine using these techniques, confirmation should be made by using more specific methods, such as a gas chromatographic-mass spectrometer technique.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term oral dosing studies with labetalol HCl for 18 months in mice and for 2 years in rats showed no evidence of carcinogenesis. Studies with labetalol HCl, using dominant lethal assays in rats and mice, and exposing microorganisms according to modified Ames tests, showed no evidence of mutagenesis.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Teratogenic studies were performed with labetalol in rats and rabbits at oral doses up to approximately six and four times the maximum recommended human dose (MRHD), respectively. No reproducible evidence of fetal malformations was observed. Increased fetal resorptions were seen in both species at doses approximating the MRHD. A teratology study performed with labetalol in rabbits at intravenous doses up to 1.7 times the MRHD revealed no evidence of drug-related harm to the fetus. There are no adequate and well controlled studies in pregnant women. Labetalol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Hypotension, bradycardia, hypoglycemia, and respiratory depression have been reported in infants of mothers who were treated with labetalol HCl for hypertension during pregnancy. Oral administration of labetalol to rats during late gestation through weaning at doses of two to four times the MRHD caused a decrease in neonatal survival.
Labor and Delivery
Labetalol HCl given to pregnant women with hypertension did not appear to affect the usual course of labor and delivery.
Nursing Mothers
Small amounts of labetalol (approximately 0.004% of the maternal dose) are excreted in human milk. Caution should be exercised when labetalol HCl tablets are administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Elderly Patients
As in the general population, some elderly patients (60 years of age or older) have experienced orthostatic hypotension, dizziness, or lightheadedness during treatment with labetalol. Because elderly patients are generally more likely than younger patients to experience orthostatic symptoms, they should be cautioned about the possibility of such side effects during treatment with labetalol.
-
ADVERSE REACTIONS
Most adverse effects are mild and transient and occur early in the course of treatment. In controlled clinical trials of 3 to 4 months' duration, discontinuation of labetalol HCl tablets due to one or more adverse effects was required in 7% of all patients. In these same trials, other agents with solely beta-blocking activity used in the control groups led to discontinuation in 8% to 10% of patients, and a centrally acting alpha-agonist led to discontinuation in 30% of patients.
The incidence rates of adverse reactions listed in the following table were derived from multicenter controlled clinical trials comparing labetalol HCl, placebo, metoprolol, and propranolol over treatment periods of 3 and 4 months. Where the frequency of adverse effects for labetalol HCl and placebo is similar, causal relationship is uncertain. The rates are based on adverse reactions considered probably drug related by the investigator. If all reports are considered, the rates are somewhat higher (e.g., dizziness 20%, nausea 14%, fatigue 11%), but the overall conclusions are unchanged.
Labetalol
HCl
(n=227)
%
Placebo
(n=98)
%
Propranolol
(n=84)
%
Metoprolol
(n=49)
%
Body as a Whole
Fatigue
5
0
12
12
Asthenia
1
1
1
0
Headache
2
1
0
2
Gastrointestinal
Nausea
6
1
1
2
Vomiting
<1
0
0
0
Dyspepsia
3
1
1
0
Abdominal pain
0
0
1
2
Diaarrhea
<1
0
2
0
Taste distortion
1
0
0
0
Central and Peripheral Nervous Systems
Dizziness
11
3
4
4
Paresthesias
<1
0
0
0
Drowsiness
<1
2
2
2
Autonomic Nervous System
Nasal
stuffiness
3
0
0
0
Ejaculation
failure
2
0
0
0
Impotence
1
0
1
3
Increased
sweating
<1
0
0
0
Cardiovascular
Edema
1
0
0
0
Postural
hypotension
1
0
0
0
Bradycardia
0
0
5
12
Respiratory
Dyspnea
2
0
1
2
Skin
Rash
1
0
0
0
Special Senses
Vision
abnormality
1
0
0
0
Vertigo
2
1
0
0
The adverse effects were reported spontaneously and are representative of the incidence of adverse effects that may be observed in a properly selected hypertensive patient population, i.e. a group excluding patients with bronchospastic disease, overt congestive heart failure, or other contraindications to beta-blocker therapy.
Clinical trials also included studies utilizing daily doses up to 2,400 mg in more severely hypertensive patients. Certain of the side effects increased with increasing dose, as shown in the following table that depicts the entire U.S. therapeutic trials data base for adverse reactions that are clearly or possibly dose related.
Labetalol HCl
Daily Dose
(mg)
200
300
400
600
800
900
1,200
1,600
2,400
Number of
patients
522
181
606
608
503
117
411
242
175
Dizzines(%)
2
3
3
3
5
1
9
13
16
Fatigue
2
1
4
4
5
3
7
6
10
Nausea
<1
0
1
2
4
0
7
11
19
Vomiting
0
0
<1
<1
<1
0
1
2
3
Dyspepsia
1
0
2
1
1
0
2
2
4
Paresthesia
2
0
2
2
1
1
2
5
5
Nasal
stuffiness
1
1
2
2
2
2
4
5
6
Ejaculation
failure
0
2
0
2
3
0
4
3
5
Impotence
1
1
1
1
2
4
3
4
3
Edema
1
0
1
1
1
0
1
2
2
In addition, a number of other less common adverse events have been reported:
Body as a Whole
Fever
Cardiovascular
Hypotension, and rarely, syncope, bradycardia, heart block.
Central and Peripheral Nervous Systems
Paresthesia, most frequently described as scalp tingling. In most cases, it was mild and transient and usually occurred at the beginning of
treatment.Collagen Disorders
Systemic lupus erythematosus, positive antinuclear factor.
Eyes
Dry eyes
Immunological system
Antimitochondrial antibodies
Liver and Biliary System
Hepatic necrosis, hepatitis, cholestatic jaundice, elevated liver function tests.
Musculoskeletal System
Muscle cramps, toxic myopathy.
Respiratory System
Bronchospasm.
Skin and Appendages
Rashes of various types, such as generalized maculopapular, lichenoid, urticarial, bullous lichen planus, psoriasiform, and facial erythema; Peyronie's disease; reversible alopecia.
Urinary System
Difficulty in micturition, including acute urinary bladder retention.
Hypersensitivity
Rare reports of hypersensitivity (e.g., rash, urticaria, pruritus, angioedema, dyspnea) and anaphylactoid reactions.
Following approval for marketing in the United Kingdom, a monitored release survey involving approximately 6,800 patients was conducted for further safety and efficacy evaluation of this product. Results of this survey indicate that the type, severity, and incidence of adverse effects were comparable to those cited above.
Potential Adverse Effects
In addition, other adverse effects not listed above have been reported with other beta-adrenergic blocking agents.
Central Nervous System
Reversible mental depression progressing to catatonia, an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on psychometrics.
Cardiovascular
Intensification of A-V block (see CONTRAINDICATIONS).
Allergic
Fever combined with aching and sore throat, laryngospasm, respiratory distress.
Hematologic
Agranulocytosis, thrombocytopenic or nonthrombocytopenic purpura.
Gastrointestinal
Mesenteric artery thrombosis, ischemic colitis.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with labetalol HCl.
Clinical Laboratory Tests
There have been reversible increases of serum transaminases in 4% of patients treated with labetalol and tested and, more rarely, reversible increases in blood urea.
-
OVERDOSAGE
Overdosage with labetalol HCl causes excessive hypotension that is posture sensitive and, sometimes, excessive bradycardia. Patients should be placed supine and their legs raised if necessary to improve the blood supply to the brain. If overdosage with labetalol HCl follows oral ingestion, gastric lavage or pharmacologically induced emesis (using syrup of ipecac) may be useful for removal of the drug shortly after ingestion. The following additional measures should be employed if necessary:
Excessive bradycardia - administer atropine or epinephrine.
Cardiac failure - administer a digitalis glycoside and a diuretic. Dopamine or dobutamine may also be useful.
Hypotension- administer vasopressors, e.g., norepinephrine. There is pharmacological evidence that norepinephrine may be the drug of choice.
Bronchospasm- administer epinephrine and/or an aerosolized beta 2-agonist.
Seizures- administer diazepam.
In severe beta-blocker overdose resulting in hypotension and/or bradycardia, glucagon has been shown to be effective when administered in large doses (5 mg to 10 mg rapidly over 30 seconds, followed by continuous infusion of 5 mg per hour that can be reduced as the patient improves).
Neither hemodialysis nor peritoneal dialysis removes a significant amount of labetalol HCl from the general circulation (<1%).
The oral LD 50 value of labetalol HCl in the mouse is approximately 600 mg/kg and in the rat is >2g/kg. The intravenous LD 50 in these species is 50 mg/kg to 60 mg/kg.
-
DOSAGE AND ADMINISTRATION
DOSAGE MUST BE INDIVIDUALIZED. The recommended initial dosage is 100 mg twice daily whether used alone or added to a diuretic regimen. After 2 or 3 days, using standing blood pressure as an indicator, dosage may be titrated in increments of 100 mg b.i.d. every 2 or 3 days. The usual maintenance dosage of labetalol HCl is between 200 mg and 400 mg twice daily.
Since the full antihypertensive effect of labetalol HCl is usually seen within the first 1 to 3 hours of the initial dose or dose increment, the assurance of a lack of an exaggerated hypotensive response can be clinically established in the office setting. The antihypertensive effects of continued dosing can be measured at subsequent visits, approximately 12 hours after a dose, to determine whether further titration is necessary.
Patients with severe hypertension may require from 1,200 mg to 2,400 mg per day, with or without thiazide diuretics. Should side effects (principally nausea or dizziness) occur with these doses administered twice daily, the same total daily dose administered three times daily may improve tolerability and facilitate further titration. Titration increments should not exceed 200 mg twice daily.
When a diuretic is added, an additive antihypertensive effect can be expected. In some cases this may necessitate a labetalol HCl dosage adjustment. As with most antihypertensive drugs, optimal dosages of labetalol HCl tablets are usually lower in patients also receiving a diuretic.
When transferring patients from other antihypertensive drugs, labetalol HCl tablets should be introduced as recommended and the dosage of the existing therapy progressively decreased.
Elderly Patients
As in the general patient population, labetalol therapy may be initiated at 100 mg twice daily and titrated upwards in increments of 100 mg twice daily as required for control of blood pressure. Since some elderly patients eliminate labetalol more slowly, however, adequate control of blood pressure may be achieved at a lower maintenance dosage compared to the general population. The majority of elderly patients will require between 100 mg and 200 mg twice daily.
-
HOW SUPPLIED
Labetalol Hydrochloride Tablets, USP 100 mg are available as white to off-white, round biconvex tablets, debossed “AC 370” on one side and bisected on other side, packaged in bottles of 100 and 500 tablets.
Bottles of 100: 51407-614-01
Bottles of 500: 51407-614-05
Labetalol Hydrochloride Tablets, USP 200 mg are available as white to off-white, round biconvex tablets, debossed “AC 371” on one side and bisected on other side, packaged in bottles of 100 and 500 tablets.
Bottles of 100: 51407-615-01
Bottles of 500: 51407-615-05
Labetalol Hydrochloride Tablets, USP 300 mg are available as white to off-white, round biconvex tablets, debossed “AC 372” on one side and plain on other side, packaged in bottles of 100 tablets.
Bottles of 100: 51407-616-01
Labetalol Hydrochloride Tablets, USP should be stored at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
To report SUSPECTED ADVERSE REACTIONS, contact Biocon Pharma Inc., at 1-866-924-6266 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Manufactured for:
Biocon Pharma Inc.,
Iselin, New Jersey,
USA
Manufactured by:
Appco Pharma LLC
Piscataway, NJ 08854
Rev. 04/2021
BPCM0128/01
200545
Marketed/Packaged by:
GSMS, Inc.
Camarillo, CA USA 93012
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-614(NDC:70377-060) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 100 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (White to off-white) Score 2 pieces Shape ROUND (Round Biconvex Tablet) Size 8mm Flavor Imprint Code AC;370 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-614-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 2 NDC:51407-614-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209603 06/20/2018 LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-615(NDC:70377-061) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 200 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (White to off-white) Score 2 pieces Shape ROUND (Round Biconvex Tablet) Size 10mm Flavor Imprint Code AC;371 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-615-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 2 NDC:51407-615-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209603 06/20/2018 LABETALOL HYDROCHLORIDE
labetalol hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:51407-616(NDC:70377-062) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LABETALOL HYDROCHLORIDE (UNII: 1GEV3BAW9J) (LABETALOL - UNII:R5H8897N95) LABETALOL HYDROCHLORIDE 300 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white (White to off-white) Score no score Shape ROUND (Round Biconvex Tablet) Size 11mm Flavor Imprint Code AC;372 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51407-616-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/03/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209603 06/20/2018 Labeler - Golden State Medical Supply, Inc. (603184490) Establishment Name Address ID/FEI Business Operations Golden State Medical Supply, Inc. 603184490 relabel(51407-614, 51407-615, 51407-616) , repack(51407-614, 51407-615, 51407-616)






