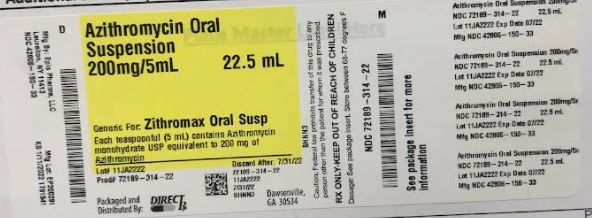
Label: AZITHROMYCIN powder, for suspension
- NDC Code(s): 72189-314-22
- Packager: DirectRx
- This is a repackaged label.
- Source NDC Code(s): 42806-150
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGEAzithromycin for oral suspension USP is a macrolide antibacterial drug indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated ...
-
DOSAGE & ADMINISTRATION2.1 Adult Patients - [see INDICATIONS AND USAGE (1.1) and CLINICAL PHARMACOLOGY (12.3)] Infection* Recommended Dose/Duration of Therapy - Community-acquired pneumonia - Pharyngitis/tonsillitis ...
-
DOSAGE FORMS & STRENGTHSAzithromycin for oral suspension USP after constitution contains a banana-cherry flavored suspension. Azithromycin for oral suspension USP is supplied to provide 100 mg/5 mL or 200 mg/5 mL ...
-
CONTRAINDICATIONS4.1 Hypersensitivity - Azithromycin for oral suspension is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide drug. 4.2 Hepatic ...
-
WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Stevens-Johnson syndrome, and toxic epidermal necrolysis have been reported ...
-
ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to ...
-
DRUG INTERACTIONS7.1 Nelfinavir - Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of ...
-
USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any drug-associated ...
-
OVERDOSAGEAdverse reactions experienced at higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea, and vomiting. In the event of overdosage, general ...
-
DESCRIPTIONAzithromycin for oral suspension USP contains the active ingredient azithromycin monohydrate, USP, a macrolide antibacterial drug, for oral administration. Azithromycin has the chemical name ...
-
CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azithromycin is a macrolide antibacterial drug. [see MICROBIOLOGY (12.4)] 12.2 Pharmacodynamics - Based on animal models of infection, the antibacterial activity of ...
-
NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential in ...
-
CLINICAL STUDIES14.1 Adult Patients - Acute Bacterial Exacerbations of Chronic Bronchitis - In a randomized, double-blind controlled clinical trial of acute exacerbation of chronic bronchitis (AECB), azithromycin (500 ...
-
HOW SUPPLIEDAzithromycin for oral suspension USP after constitution contains a banana-cherry flavored suspension. Azithromycin for oral suspension USP is supplied to provide 100 mg/5 mL or 200 mg/5 mL ...
-
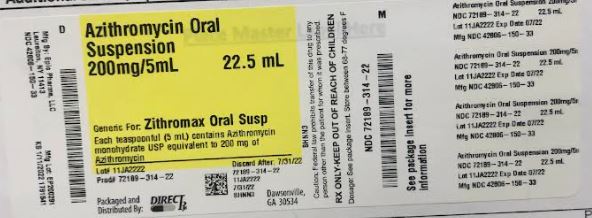
PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCEProduct Information