Label: NAFTIFINE HYDROCHLORIDE cream
- NDC Code(s): 51672-1362-1, 51672-1362-2, 51672-1362-3, 51672-1362-8
- Packager: Taro Pharmaceuticals U.S.A., Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated May 25, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx ONLY
-
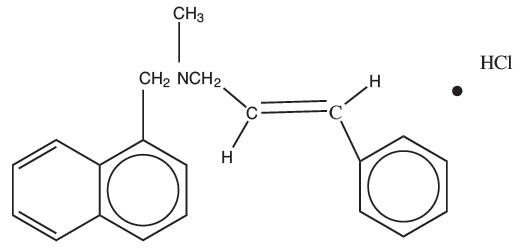
DESCRIPTIONNaftifine Hydrochloride Cream USP, 1% contains the synthetic, broad-spectrum, antifungal agent naftifine hydrochloride. Naftifine Hydrochloride Cream USP, 1% is for topical use only. CHEMICAL ...
-
CLINICAL PHARMACOLOGYNaftifine hydrochloride is a synthetic allylamine derivative. The following in vitro data are available, but their clinical significance is unknown. Naftifine hydrochloride has been shown to ...
-
INDICATIONS AND USAGENaftifine Hydrochloride Cream USP, 1% is indicated for the topical treatment of tinea pedis, tinea cruris and tinea corporis caused by the organisms Trichophyton rubrum, Trichophyton ...
-
CONTRAINDICATIONSNaftifine Hydrochloride Cream USP, 1% is contraindicated in individuals who have shown hypersensitivity to any of its components.
-
WARNINGSNaftifine Hydrochloride Cream USP, 1% is for topical use only and not for ophthalmic use.
-
PRECAUTIONSGeneral - Naftifine Hydrochloride Cream USP, 1% is for external use only. If irritation or sensitivity develops with the use of Naftifine Hydrochloride Cream USP, 1%, treatment should be ...
-
ADVERSE REACTIONSDuring clinical trials with Naftifine Hydrochloride Cream USP, 1%, the incidence of adverse reactions was as follows: burning/stinging (6%), dryness (3%), erythema (2%), itching (2%), local ...
-
DOSAGE AND ADMINISTRATIONA sufficient quantity of Naftifine Hydrochloride Cream USP, 1% should be gently massaged into the affected and surrounding skin areas once a day. The hands should be washed after application. If ...
-
HOW SUPPLIEDNaftifine Hydrochloride Cream USP, 1% is supplied in the following sizes: 15 g - NDC 51672-1362-1 (tube) 30 g - NDC 51672-1362-2 (tube) 60 g - NDC 51672-1362-3 (tube) 90 g - NDC 51672-1362-8 ...
-
SPL UNCLASSIFIED SECTIONManufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 - Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532 - TARO is a registered trademark of Taro ...
-
PRINCIPAL DISPLAY PANEL - 90 g Tube CartonNDC 51672-1362-8 - 90 g - Naftifine Hydrochloride - Cream USP, 1% FOR TOPICAL USE ONLY. NOT FOR OPHTHALMIC USE. Rx only - TARO - Keep this and all medications out of the reach of children.
-
INGREDIENTS AND APPEARANCEProduct Information