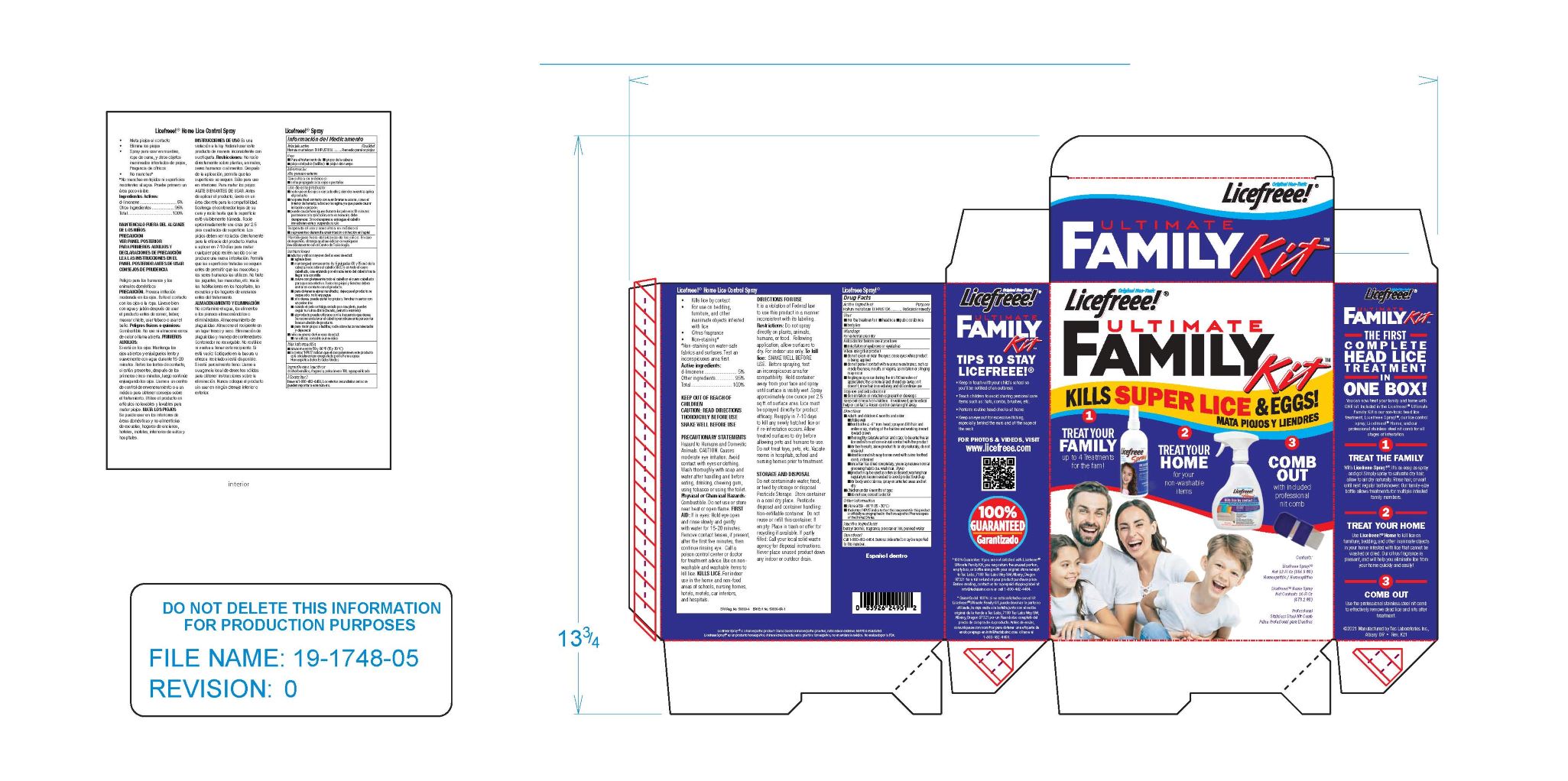
Label: LICEFREEE ULTIMATE FAMILY KIT- head lice treatment kit
- NDC Code(s): 51879-300-12
- Packager: Tec Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 2, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
WHEN USING
When using this product
- do not use in or near the eyes; close eyes when product is being applied
- do not permit contact with mucus membranes, such as inside the nose, mouth or vagina, as irritation or stinging may occur
- tingling may occur during the first 10 minutes of application; this is normal and should go away; if it doesn't, rinse hair immediately and discontinue use
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
DOSAGE & ADMINISTRATION
Directions
- Adults and children 6 months and older:
- shake well before each use
- hold bottle 4-6 " from head; spray on DRY hair and entire scalp, starting at the hairline and working inward toward the crown
- thoroughly saturate all hair and scalp; to be effective all lice and nits must come in full contact with the product
- dead lice and nits may be removed with a fine tooth comb, if desired
- for best results, allow product to air dry naturally, do not rinse out
- product may be used as often as desired; washing hair regularly is recommended to aviod product build-up
- for body and crab lice, spray on affected areas and let dry
- Children under 6 months of age:
- do not use; consult a doctor
- Adults and children 6 months and older:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LICEFREEE ULTIMATE FAMILY KIT
head lice treatment kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51879-300 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51879-300-12 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 02/09/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 354.9 mL Part 1 of 1 LICEFREEE
head lice treatment sprayProduct Information Item Code (Source) NDC:51879-180 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 1 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZYL ALCOHOL (UNII: LKG8494WBH) POLOXAMER 188 (UNII: LQA7B6G8JG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 354.9 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/09/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/09/2022 Labeler - Tec Laboratories, Inc. (083647792) Establishment Name Address ID/FEI Business Operations Tec Laboratories, Inc. 083647792 manufacture(51879-300)