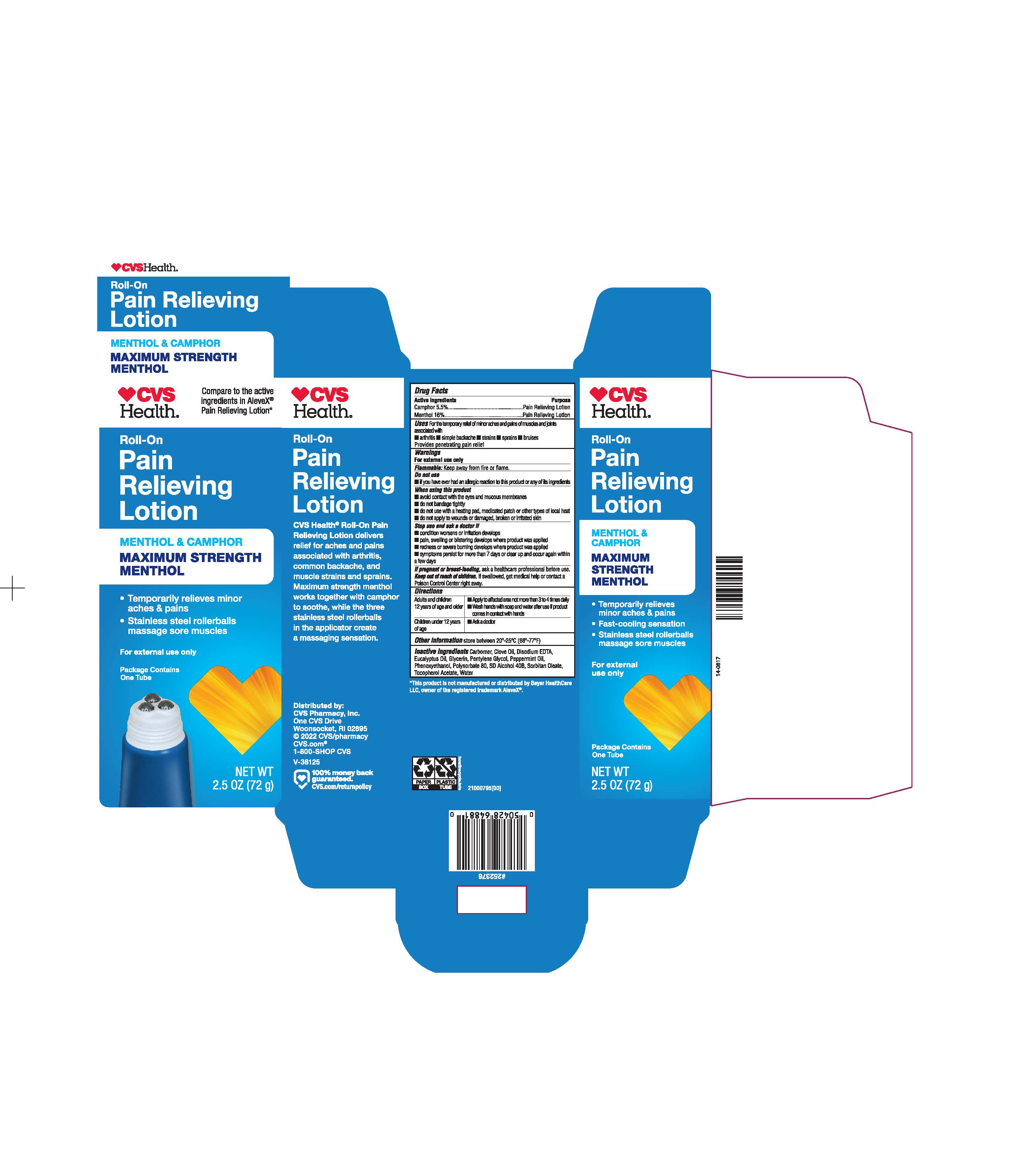
Label: CVS PAIN RELIEVING- camphor 5.5% menthol 16% lotion
- NDC Code(s): 51316-885-16
- Packager: CVS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
For extranal use only
avoid contact with eyes and mucous membranes
do not bandage tightly
do not use with a heating pad, medicated patch or other of local heat
do not apply to wounds or damaged, broken or irritated skin
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CVS PAIN RELIEVING
camphor 5.5% menthol 16% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51316-885 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 16 mg in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PENTYLENE GLYCOL (UNII: 50C1307PZG) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) PHENOXYETHANOL (UNII: HIE492ZZ3T) EUCALYPTUS OIL (UNII: 2R04ONI662) PEPPERMINT OIL (UNII: AV092KU4JH) CLOVE OIL (UNII: 578389D6D0) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51316-885-16 72 g in 1 TUBE; Type 0: Not a Combination Product 12/19/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/19/2022 Labeler - CVS (062312574)