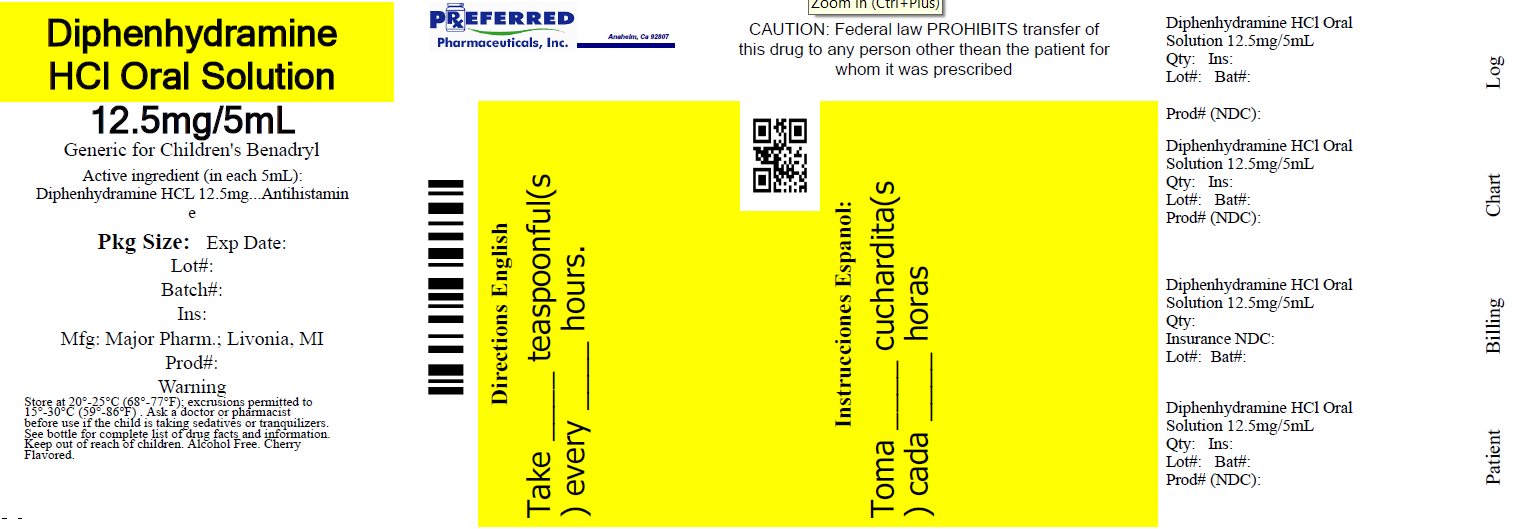
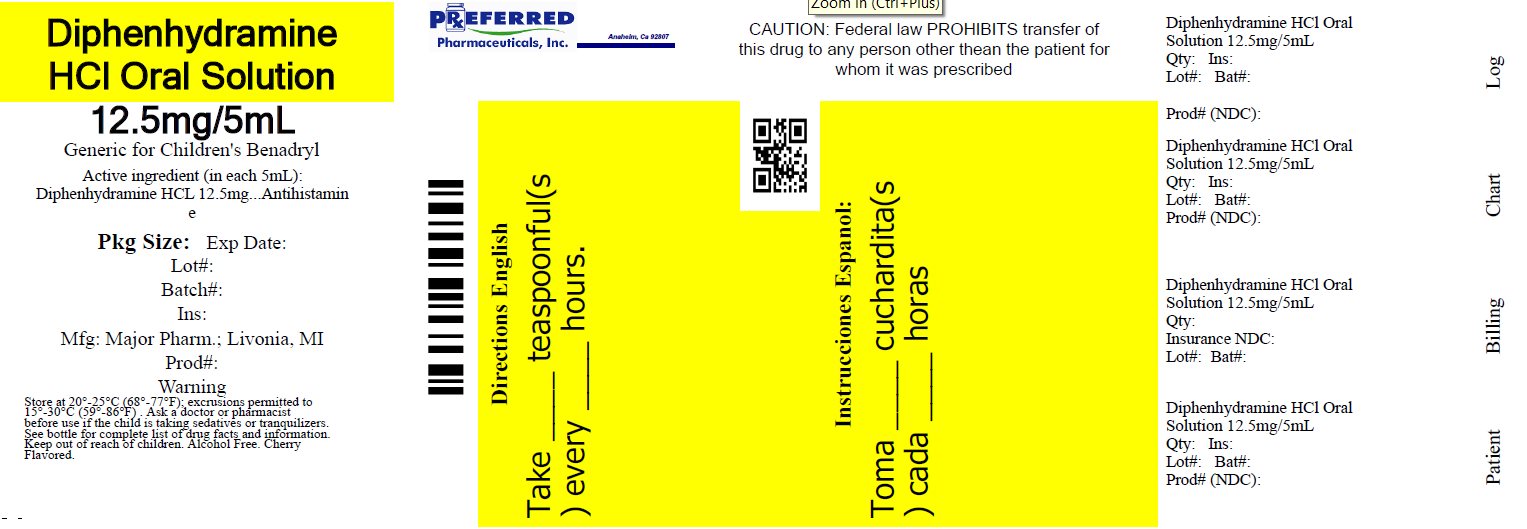
Label: DIPHENHYDRAMINE HCL solution
- NDC Code(s): 68788-8274-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 0904-6985
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient (in each teaspoonful (5 mL))
Diphenhydramine HCl 12.5 mg
-
Purpose
Antihistamine
-
Uses
• temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: o - runny nose - o - sneezing - o - itchy, watery eyes - o - itching of the nose or throat
-
Warnings
Do not use - • to make a child sleepy - • with any other product containing diphenhydramine, even one used on skin - Ask a doctor before use if you have - • a breathing problem such as ...
-
Directions
• do not take more than 6 doses in 24 hours - • mL = milliliter; FL OZ = fluid ounce - • find right dose on chart below - • take every 4 to 6 hours, or as directed by a ...
-
Other information
• each teaspoonful (5 mL) contains: sodium 5 mg - • store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F) • use by expiration date on package
-
Inactive ingredients
anhydrous citric acid, D&C red #33, FD&C red #40, flavors, glycerin, high fructose corn syrup, purified water, sodium benzoate, sodium chloride, sodium citrate dihydrate, sucrose
-
Questions or comments?
(800) 616-2471 - Relabeled By: Preferred Pharmaceuticals Inc.
-
Principal display panel
MAJOR® NDC 68788-8274-1 - Relabeled By: Preferred Pharmaceuticals Inc. Diphenhydramine HCl - Oral Solution - Antihistamine - 12.5 mg/5 mL - Institutional Dispensing only - Cherry ...
-
INGREDIENTS AND APPEARANCEProduct Information