Label: AZITHROMYCIN injection, powder, lyophilized, for solution
- NDC Code(s): 0409-0144-11, 0409-0144-21
- Packager: Hospira, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Azithromycin safely and effectively. See full prescribing information for Azithromycin. AZITHROMYCIN for injection, for intravenous ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEAzithromycin for Injection, USP is a macrolide antibacterial drug indicated for the treatment of patients with infections caused by susceptible strains of the designated microorganisms in the ...
-
2 DOSAGE AND ADMINISTRATION[see Indications and Usage (1) and Clinical Pharmacology (12.3)]. 2.1 Community-Acquired Pneumonia - The recommended dose of Azithromycin for Injection, USP for the treatment of adult patients ...
-
3 DOSAGE FORMS AND STRENGTHSAzithromycin for Injection is supplied as white to off-white lyophilized powder in a single-dose ADD-Vantage® vial equivalent to 500 mg of azithromycin for intravenous administration.
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Azithromycin for Injection is contraindicated in patients with known hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide drugs. 4.2 Hepatic ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypersensitivity - Serious allergic reactions, including angioedema, anaphylaxis, and dermatologic reactions including Acute Generalized Exanthematous Pustulosis (AGEP), Stevens-Johnson ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in labeling: • Hypersensitivity [see Warnings and Precautions (5.1)] • Hepatotoxicity [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Nelfinavir - Co-administration of nelfinavir at steady-state with a single oral dose of azithromycin resulted in increased azithromycin serum concentrations. Although a dose adjustment of ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data from published literature and postmarketing experience over several decades with azithromycin use in pregnant women have not identified any ...
-
10 OVERDOSAGEAdverse reactions experienced in higher than recommended doses were similar to those seen at normal doses particularly nausea, diarrhea and vomiting. In the event of overdosage, general ...
-
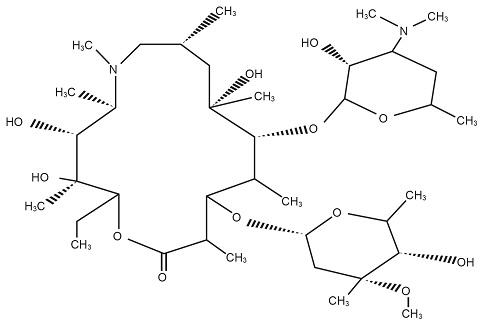
11 DESCRIPTIONAzithromycin for Injection, USP contains the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for intravenous injection. Azithromycin has the chemical name ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Azithromycin is a macrolide antibacterial drug [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Based on animal models of infection, the antibacterial activity of ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Azithromycin has shown no mutagenic potential ...
-
14 CLINICAL STUDIES14.1 Community-Acquired Pneumonia - In a controlled trial of community-acquired pneumonia performed in the U.S., azithromycin (500 mg as a single daily dose by the intravenous route for 2 to 5 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGAzithromycin for Injection, USP is supplied as white to off-white lyophilized powder in a single-dose ADD-Vantage® vial equivalent to 500 mg of azithromycin for intravenous administration. These ...
-
17 PATIENT COUNSELING INFORMATIONPatients should be informed of the following serious and potentially serious adverse reactions that have been associated with Azithromycin for Injection: Diarrhea: Inform patients that diarrhea ...
-
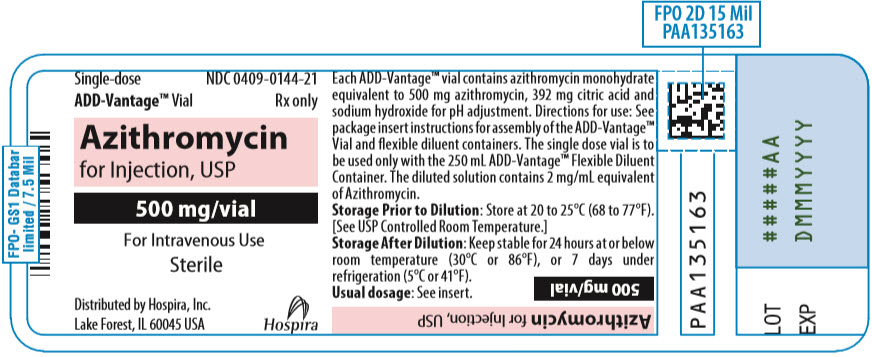
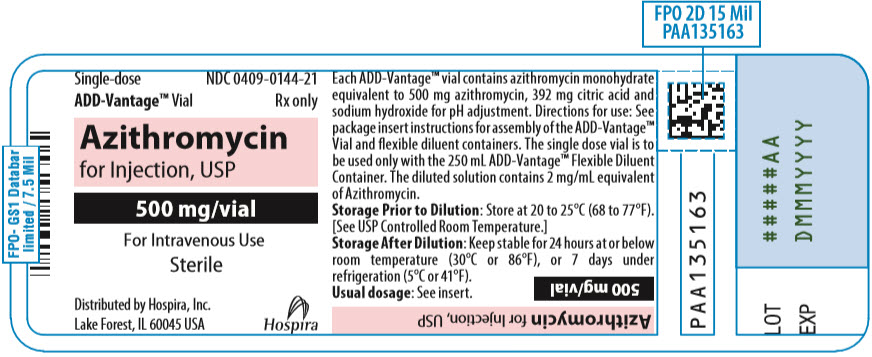
PRINCIPAL DISPLAY PANEL - 500 mg Vial LabelSingle-dose - ADD-Vantage® Vial - NDC 0409-0144-21 - Rx only - Azithromycin - for Injection, USP - 500 mg/vial - For Intravenous Use - Sterile - Distributed by Hospira, Inc. Lake Forest, IL 60045 USA - Hospira
-
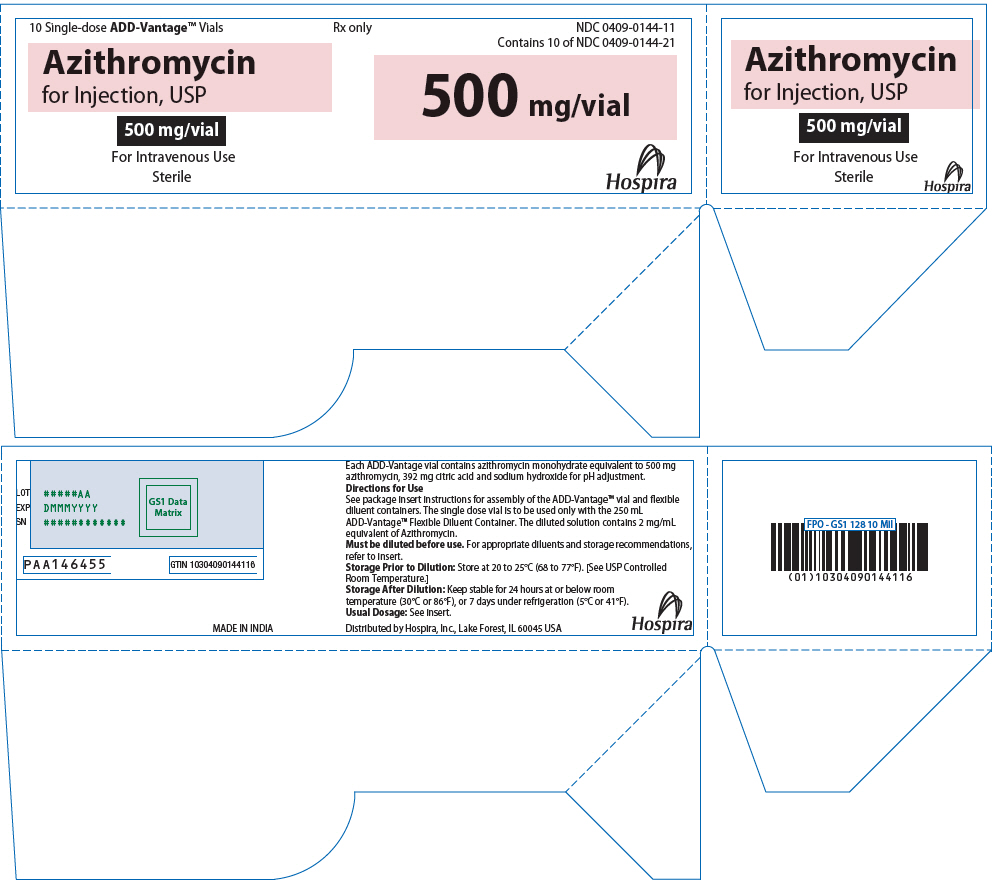
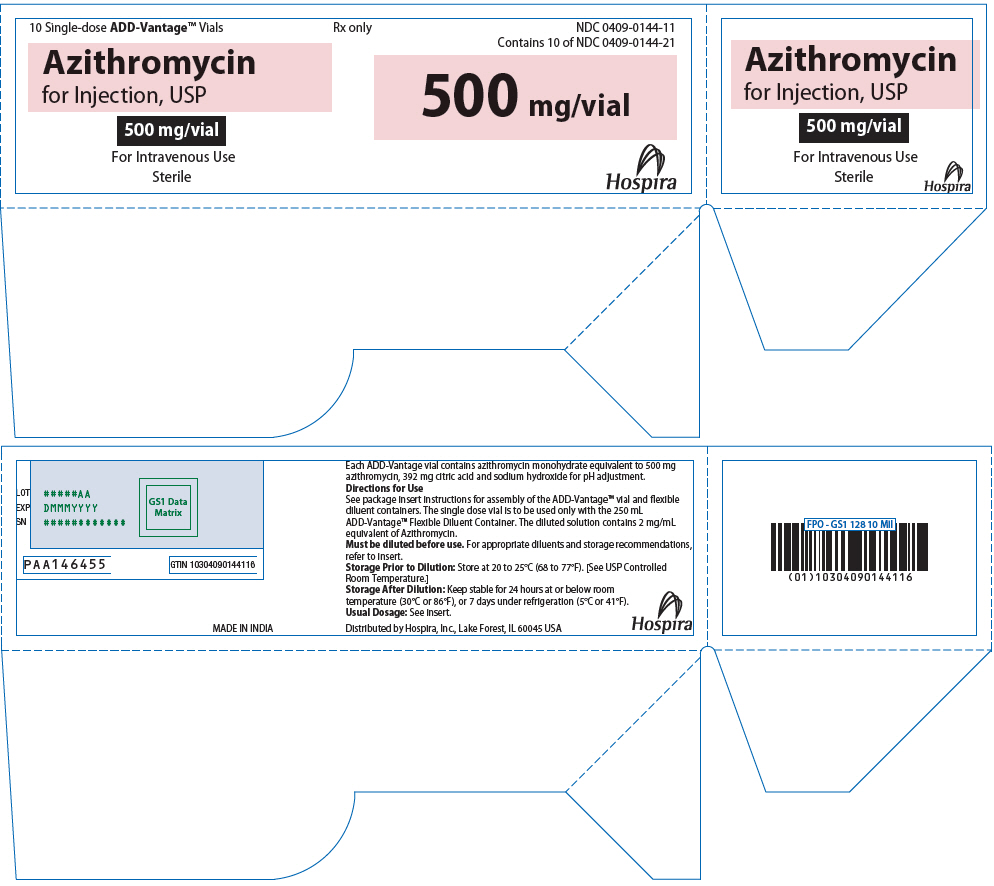
PRINCIPAL DISPLAY PANEL - 500 mg Vial Tray10 Single-dose ADD-Vantage® Vials - Rx only - NDC 0409-0144-11 - Contains 10 of NDC 0409-0144-21 - Azithromycin - for Injection, USP - 500 mg/vial - For Intravenous Use - Sterile - 500 mg/vial - Hospira
-
INGREDIENTS AND APPEARANCEProduct Information