Label: LEVETIRACETAM injection, solution
- NDC Code(s): 25021-793-82, 25021-794-82, 25021-795-82
- Packager: Sagent Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 18, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use LEVETIRACETAM IN SODIUM CHLORIDE INJECTION safely and effectively. See full prescribing information for LEVETIRACETAM IN SODIUM ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
1.1 Partial-Onset Seizures - Levetiracetam in Sodium Chloride Injection is indicated as adjunctive therapy in the treatment of partial-onset seizures in adults with epilepsy. 1.2 Myoclonic ...
-
2 DOSAGE AND ADMINISTRATION
2.1 General Information – Administration - Levetiracetam in Sodium Chloride Injection is for intravenous infusion only. It is available in the following concentrations: three single-dose 100 mL ...
-
3 DOSAGE FORMS AND STRENGTHS
Injection: Levetiracetam in Sodium Chloride Injection is a clear, colorless solution packaged in a single-dose bag and available in three strengths: 500 mg per 100 mL (5 mg per mL): 500 mg ...
-
4 CONTRAINDICATIONS
Levetiracetam in Sodium Chloride Injection is contraindicated in patients with a hypersensitivity to levetiracetam. Reactions have included anaphylaxis and angioedema [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS
5.1 Psychiatric Reactions - In some patients levetiracetam causes behavioral abnormalities. The incidences of behavioral abnormalities in the myoclonic and primary generalized tonic-clonic ...
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more details in other sections of labeling: Psychiatric Reactions [see Warnings and Precautions (5.1)] Somnolence and Fatigue [see ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antiepileptic drugs (AEDs), including levetiracetam ...
-
10 OVERDOSAGE
10.1 Signs, Symptoms and Laboratory Findings of Acute Overdosage in Humans - The highest known dose of oral levetiracetam received in the clinical development program was 6,000 mg/day. Other ...
-
11 DESCRIPTION
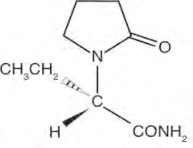
Levetiracetam in Sodium Chloride Injection is an antiepileptic drug available as a clear, colorless, sterile solution for intravenous administration. The chemical name of levetiracetam, a single ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The precise mechanism(s) by which levetiracetam exerts its antiepileptic effect is unknown. A saturable and stereoselective neuronal binding site in rat brain tissue ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Rats were dosed with levetiracetam in the diet for 104 weeks at doses of 50, 300 and 1,800 mg/kg/day. Plasma ...
-
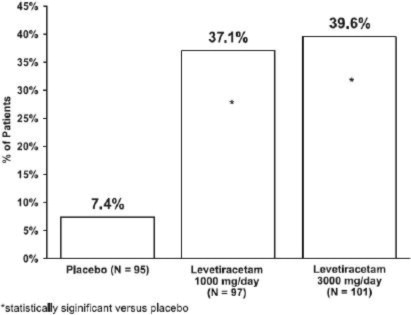
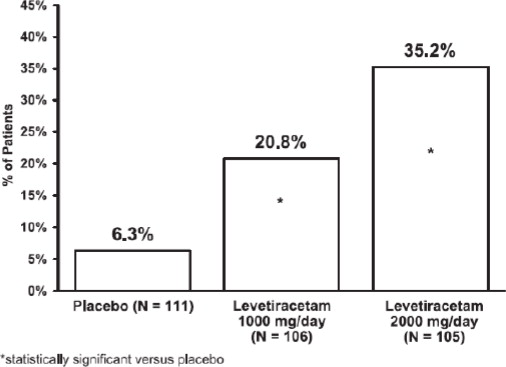
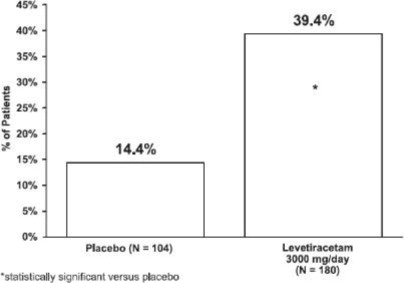
14 CLINICAL STUDIES
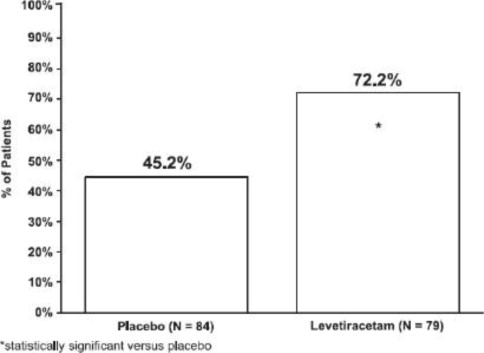
All clinical studies supporting the efficacy of levetiracetam utilized oral formulations. The finding of efficacy of levetiracetam injection is based on the results of studies using an oral ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied - Levetiracetam in Sodium Chloride Injection is supplied as follows: NDCLevetiracetam in 0.82% Sodium Chloride Injection (5 mg per mL)Package Factor - 25021-793-82 - 500 ...
-
17 PATIENT COUNSELING INFORMATION
Psychiatric Reactions and Changes in Behavior - Advise patients and their caregivers that levetiracetam may cause changes in behavior (e.g., aggression, agitation, anger, anxiety, apathy ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Bag Label - NDC 25021-793-82 - 100 mL - Levetiracetam in 0.82% Sodium Chloride Injection - 500 mg per 100 mL - (5 mg per mL) Rx only - For Intravenous Infusion ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Bag Label - NDC 25021-794-82 - 100 mL - Levetiracetam in 0.75% Sodium Chloride Injection - 1,000 mg per 100 mL - (10 mg per mL) Rx only - For Intravenous ...
-
PRINCIPAL DISPLAY PANELPACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Bag Label - NDC 25021-795-82 - 100 mL - Levetiracetam in 0.54% Sodium Chloride Injection - 1,500 mg per 100 mL - (15 mg per mL) Rx only - For Intravenous ...
-
INGREDIENTS AND APPEARANCEProduct Information