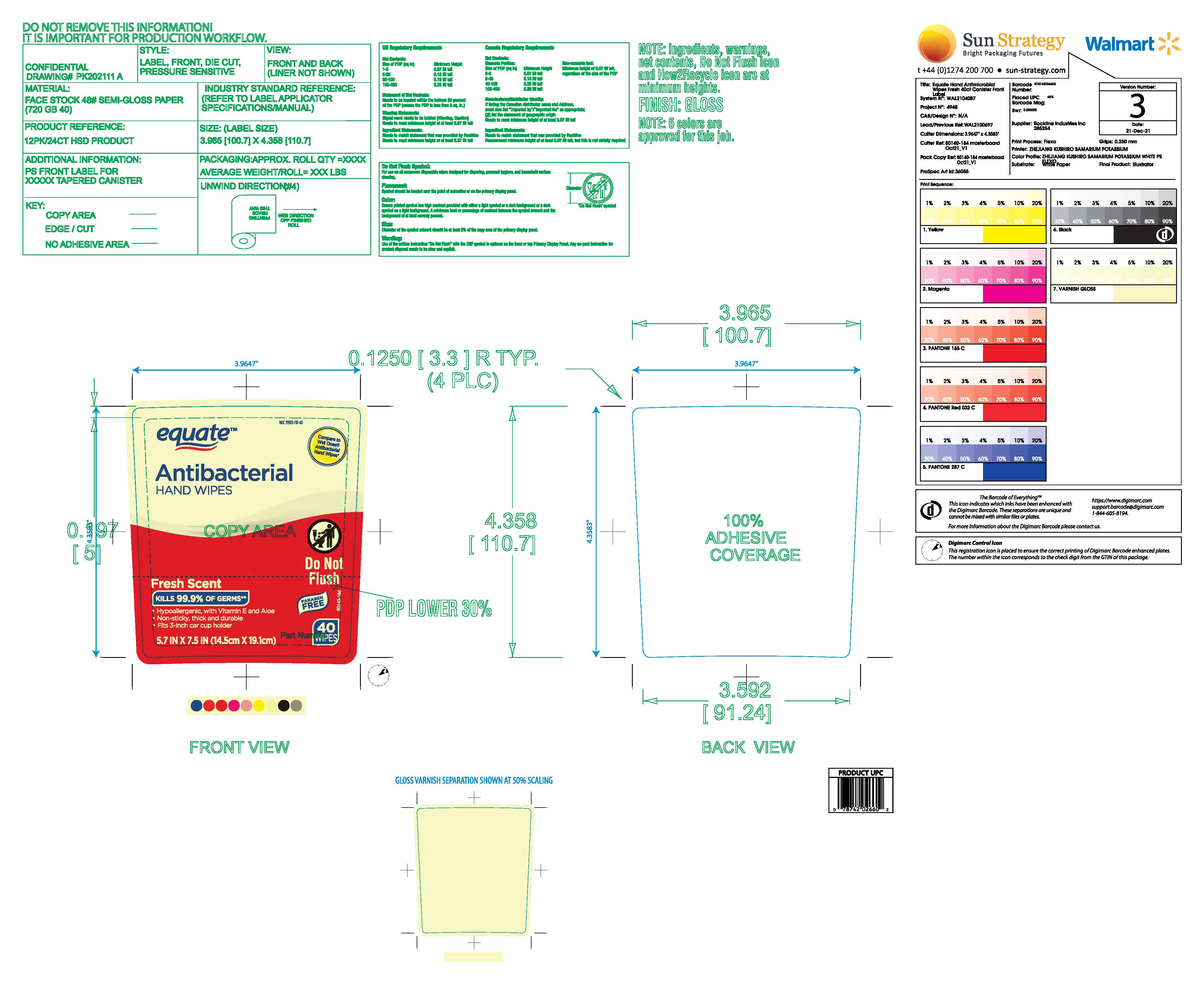
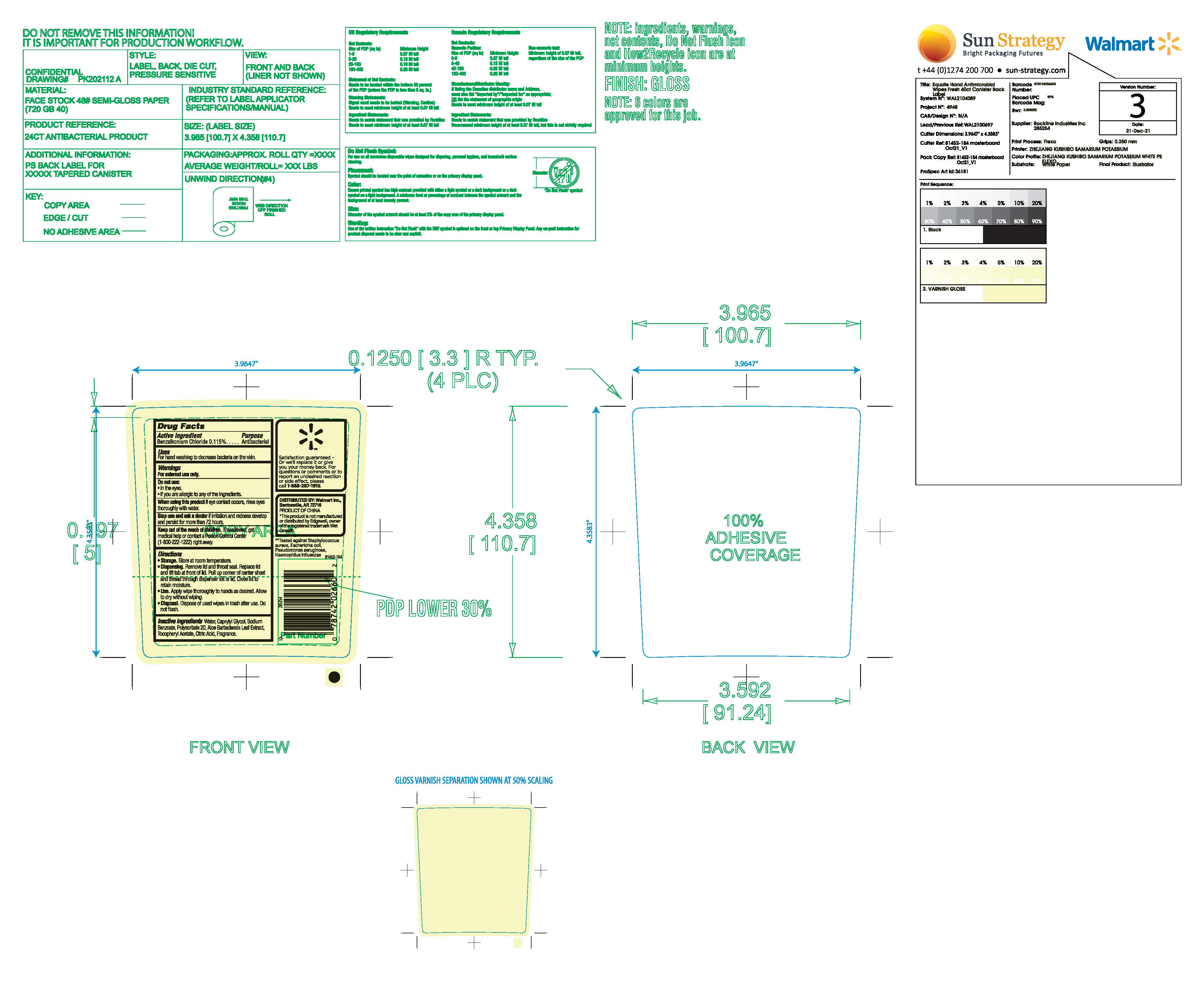
Label: EQUATE ANTIBACTERIAL FRESH HAND WIPES- benzalkonium chloride cloth
- NDC Code(s): 79903-110-40
- Packager: Walmart, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
-
Directions
- Storage, Store at room temperature
- Dispensing Remove lid and throat seal. Replace lid and lift tab at front of lid. Pull up corner of center sheet and thread through dispenser slit in lid. Close lid to retain moisture.
- Use. Apply wipe thoroughly to hands as desired. Allow to dry without wiping.
- Disposal. Dispose of used wipes in a trash receptacle after use. Do not flush.
- Inactive ingredients
- Package Label
-
INGREDIENTS AND APPEARANCE
EQUATE ANTIBACTERIAL FRESH HAND WIPES
benzalkonium chloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-110 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.115 g Inactive Ingredients Ingredient Name Strength POLYSORBATE 20 (UNII: 7T1F30V5YH) WATER (UNII: 059QF0KO0R) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-110-40 40 in 1 CANISTER; Type 0: Not a Combination Product 04/28/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 04/28/2024 Labeler - Walmart, Inc. (051957769)